Home /
Expert Answers /
Chemistry /
question-2-a-strong-base-and-a-secondary-alkyl-halide-will-undergo-an-e2-reaction-with-cyclohexa-pa895
(Solved): Question 2. A strong base and a secondary alkyl halide will undergo an E2 reaction. With cyclohexa ...
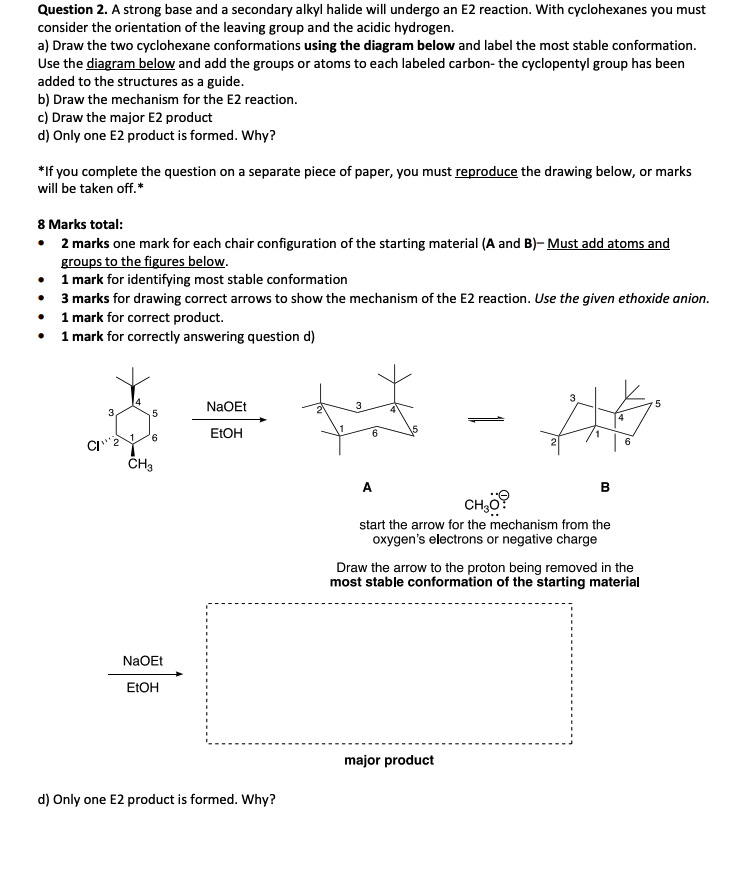
Question 2. A strong base and a secondary alkyl halide will undergo an E2 reaction. With cyclohexanes you must consider the orientation of the leaving group and the acidic hydrogen. a) Draw the two cyclohexane conformations using the diagram below and label the most stable conformation. Use the diagram below and add the groups or atoms to each labeled carbon- the cyclopentyl group has been added to the structures as a guide. b) Draw the mechanism for the E2 reaction. c) Draw the major E2 product d) Only one E2 product is formed. Why? *If you complete the question on a separate piece of paper, you must reproduce the drawing below, or marks will be taken off.* 8 Marks total: • 2 marks one mark for each chair configuration of the starting material (A and B)- Must add atoms and groups to the figures below. • 1 mark for identifying most stable conformation 3 marks for drawing correct arrows to show the mechanism of the E2 reaction. Use the given ethoxide anion. 1 mark for correct product. 1 mark for correctly answering question d) • • 3 C¹? 4 5 6 CH3 NaOEt EtOH NaOEt EtOH d) Only one E2 product is formed. Why? A B CH ?0 start the arrow for the mechanism from the oxygen's electrons or negative charge major product 6 Draw the arrow to the proton being removed in the most stable conformation of the starting material 5
Expert Answer
answer. Ring flip causes equatorial bonds to become axial bonds and axial bonds to become equatorial bonds If the chair conformers have one group in an equatorial position and one in an axial position, the more stable conformer is the one with the la