Home /
Expert Answers /
Physics /
q6-an-aluminum-calorimeter-of-mass-50-mathrm-g-has-a-volume-of-2-times-10-3-mathr-pa333
(Solved): Q6. An aluminum calorimeter of mass \( 50 \mathrm{~g} \) has a volume of \( 2 \times 10^{-3} \mathr ...
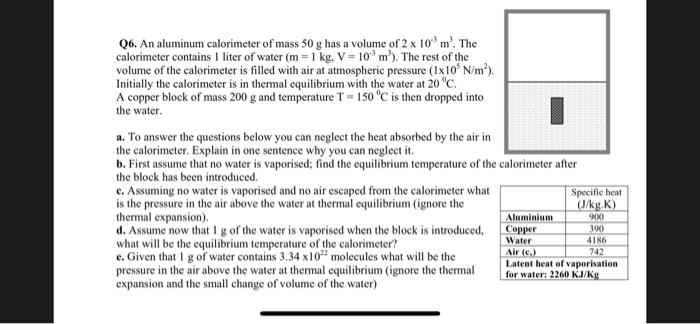
Q6. An aluminum calorimeter of mass \( 50 \mathrm{~g} \) has a volume of \( 2 \times 10^{-3} \mathrm{~m}^{3} \). The calorimeter contains 1 liter of water \( \left(\mathrm{m}=1 \mathrm{~kg}, \mathrm{~V}=10^{-3} \mathrm{~m}^{3}\right) \). The rest of the volume of the calorimeter is filled with air at atmospheric pressure \( \left(1 \times 10^{5} \mathrm{~N} / \mathrm{m}^{2}\right) \). Initially the calorimeter is in thermal equilibrium with the water at \( 20^{\circ} \mathrm{C} \). A copper block of mass \( 200 \mathrm{~g} \) and temperature \( \mathrm{T}=150^{\circ} \mathrm{C} \) is then dropped into the water. a. To answer the questions below you can neglect the heat absorbed by the air in the calorimeter. Explain in one sentence why you can neglect it. b. First assume that no water is vaporised; find the equilibrium temperature of the the block has been introduced. c. Assuming no water is vaporised and no air escaped from the calorimeter what is the pressure in the air above the water at thermal equilibrium (ignore the thermal expansion). d. Assume now that I g of the water is vaporised when the block is introduced, what will be the equilibrium temperature of the calorimeter? e. Given that \( 1 \mathrm{~g} \) of water contains \( 3.34 \times 10^{22} \) molecules what will be the pressure in the air above the water at thermal equilibrium (ignore the thermal expansion and the small change of volume of the water)
Expert Answer
Heat energy given by metal piece =m?c??T 1 =20×0.3×(100?22) =468J Heat energy gained by wat