Home /
Expert Answers /
Chemistry /
q5-calculate-the-heat-of-combustion-of-phenol-left-mathrm-c-6-mathrm-h-5-mathrm-oh-r-pa323
(Solved): Q5. Calculate the heat of combustion of phenol \( \left(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}\r ...
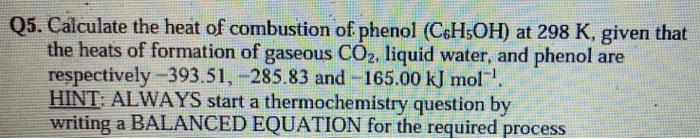
Q5. Calculate the heat of combustion of phenol \( \left(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{OH}\right) \) at \( 298 \mathrm{~K} \), given that the heats of formation of gaseous \( \mathrm{CO}_{2} \), liquid water, and phenol are respectively \( -393.51,-285.83 \) and \( -165.00 \mathrm{~kJ} \mathrm{~mol}^{-1} \). HINT: ALWAYS start a thermochemistry question by writing a BALANCED EQUATION for the required process