Home /
Expert Answers /
Chemistry /
ps6-3-answer-the-following-questions-about-the-energy-diagrams-below-a-which-diagrams-correspon-pa622
(Solved): PS6-3. Answer the following questions about the energy diagrams below. a. Which diagrams correspon ...
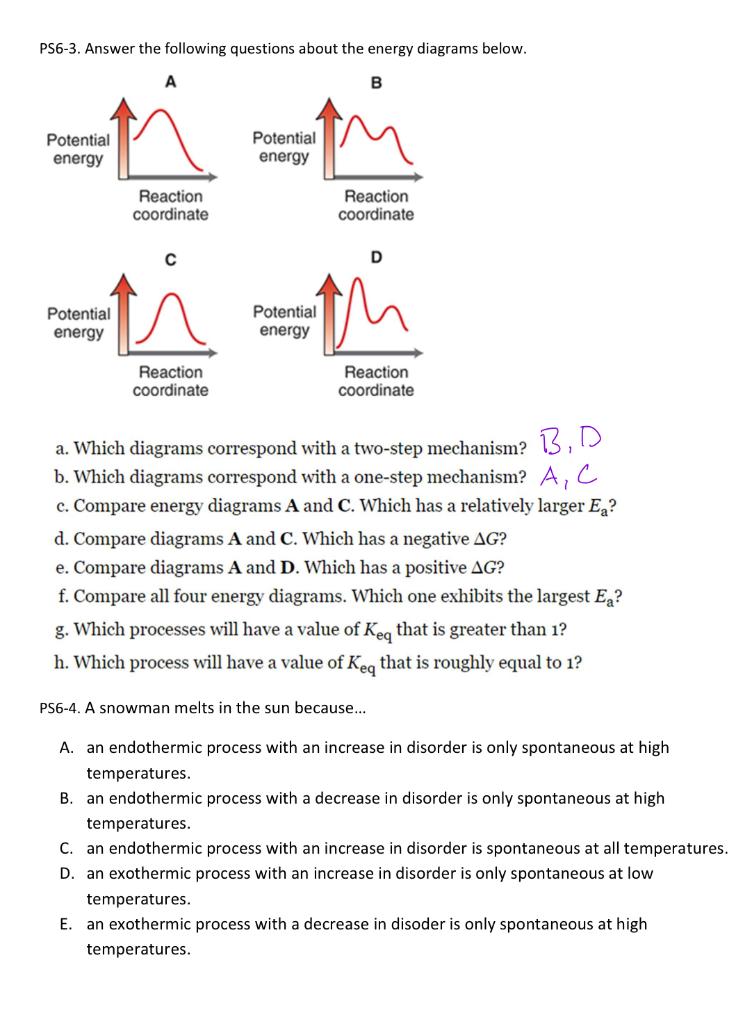
PS6-3. Answer the following questions about the energy diagrams below. a. Which diagrams correspond with a two-step mechanism? 13, D b. Which diagrams correspond with a one-step mechanism? \( A, C \) c. Compare energy diagrams \( \mathbf{A} \) and \( \mathbf{C} \). Which has a relatively larger \( E_{\mathrm{a}} \) ? d. Compare diagrams \( \mathbf{A} \) and \( \mathbf{C} \). Which has a negative \( \Delta G \) ? e. Compare diagrams \( \mathbf{A} \) and \( \mathbf{D} \). Which has a positive \( \Delta G \) ? f. Compare all four energy diagrams. Which one exhibits the largest \( E_{\mathrm{a}} \) ? g. Which processes will have a value of \( K_{\mathrm{eq}} \) that is greater than 1 ? h. Which process will have a value of \( K_{\text {eq }} \) that is roughly equal to 1 ? PS6-4. A snowman melts in the sun because... A. an endothermic process with an increase in disorder is only spontaneous at high temperatures. B. an endothermic process with a decrease in disorder is only spontaneous at high temperatures. C. an endothermic process with an increase in disorder is spontaneous at all temperatures. D. an exothermic process with an increase in disorder is only spontaneous at low temperatures. E. an exothermic process with a decrease in disoder is only spontaneous at high temperatures.