Home /
Expert Answers /
Chemical Engineering /
problem-statement-you-are-asked-to-design-a-pbr-reactor-s-to-produce-100-mathrm-kton-mathr-pa676
(Solved): Problem Statement You are asked to design a PBR reactor(s) to produce \( 100 \mathrm{kton} / \mathr ...

Problem Statement You are asked to design a PBR reactor(s) to produce \( 100 \mathrm{kton} / \mathrm{year} \) of ethylene from bioethanol (containing ethanol and water) via dehydration reaction using the following information of the catalyst with mechanism and corresponding kinetics parameters given below. The reactor is to be operated isothermally between \( 250-400^{\circ} \mathrm{C} \). The conversion of ethanol is at least 0.7. i) Calculate kinetics parameters, \( \mathrm{k} \) and \( \mathrm{K} \), in the rate expression from thermodynamic data given in Table 1. ii) Perform mass and energy balances to estimate the size of the reactor and heat duty to the reactor neglecting the pressure drop. You must assume the ratio of water to ethanol to be used. iii) Design the reactor(s) to meet the requirement below using Aspen Plus. iv) Obtain the flow rates of all species in the effluent and selectivities of products. Design Requirements In achieving the following requirements, you should consider public health, safety, and welfare, as well as global, cultural, social, environmental, and economic factors. a) The suitable scheme of the reactor: configuration, number of reactors, size of reactor(s) (tube diameter, thickness and length, schedule no., material, ) b) Heat duty of the reactor(s)
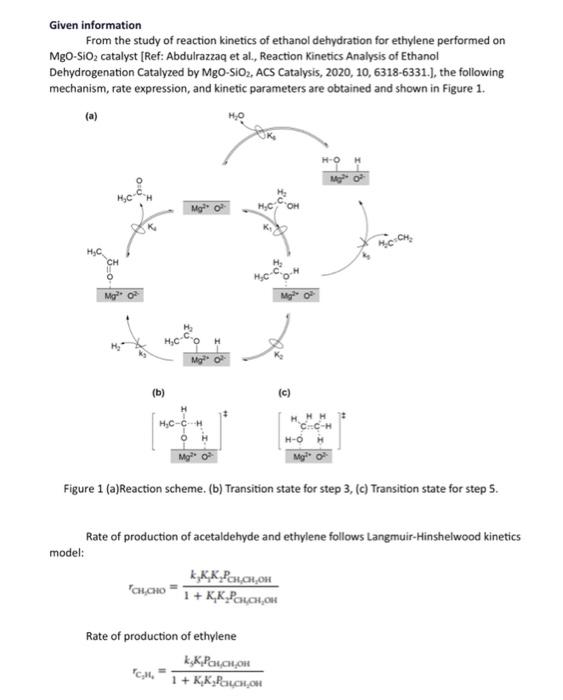
Given information From the study of reaction kinetics of ethanol dehydration for ethylene performed on \( \mathrm{MgO}_{-} \mathrm{SiO}_{2} \) catalyst [Ref: Abdulrazzaq et al., Reaction Kinetics Analysis of Ethanol Dehydrogenation Catalyzed by \( \mathrm{MgO}_{\mathrm{g}} \mathrm{SiO}_{2} \), ACS Catalysis, 2020, 10, 6318-6331.], the following mechanism, rate expression, and kinetic parameters are obtained and shown in Figure 1. (b) (C) Figure 1 (a)Reaction scheme. (b) Transition state for step 3, (c) Transition state for step 5. Rate of production of acetaldehyde and ethylene follows Langmuir-Hinshelwood kinetics model: \[ r_{\text {CHOHO }}=\frac{\mathrm{K}_{3} \mathrm{~K}_{1} \mathrm{~K}_{2} P_{\mathrm{CH}_{2} \mathrm{OH}_{2} \mathrm{OH}}}{1+\mathrm{K}_{1} \mathrm{~K}_{2} \mathrm{P}_{\mathrm{C}} \mathrm{CH}_{2} \mathrm{OH}} \] Rate of production of ethylene \[ r_{\mathrm{C}_{2} \mathrm{H}_{4}}=\frac{\mathrm{K}_{3} \mathrm{~K}_{1} P_{\mathrm{OU}} \mathrm{CH}_{3} \mathrm{OH}}{1+\mathrm{K}_{2} \mathrm{~K}_{2} \mathrm{P}_{\mathrm{Cu}} \mathrm{CH}_{2} \mathrm{OH}} \]
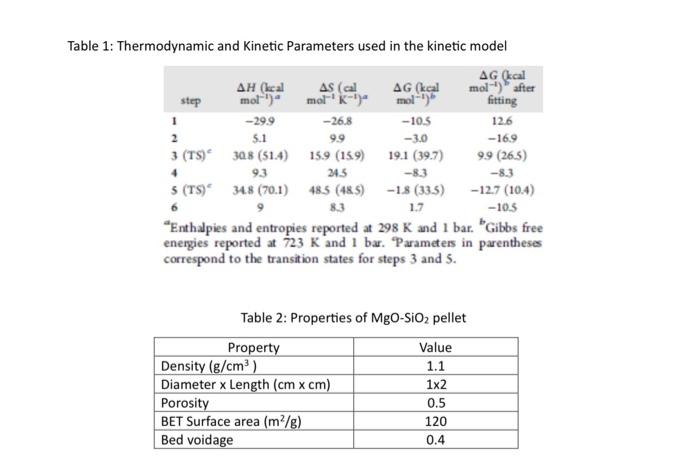
Table 1: Thermodynamic and Kinetic Parameters used in the kinetic model \( { }^{a} \) Enthalpies and entropies reported at \( 298 \mathrm{~K} \) and 1 bar. \( { }^{b} \) Gibbs free energies reported at \( 723 \mathrm{~K} \) and 1 bar. Parameters in parentheses correspond to the transition states for steps 3 and 5 . Table 2: Properties of \( \mathrm{MgO}_{-} \mathrm{SiO}_{2} \) pellet
Expert Answer
The model Langmuir hinshelwood hougen Watson are the equation which is used to defined the kinetics of chemical reactions which catalyzed by the heterogeneous cat