Home /
Expert Answers /
Chemical Engineering /
problem-7-use-the-phase-diagram-of-silicon-to-answer-the-following-questions-for-the-y-axis-of-th-pa737
(Solved): Problem 7: Use the phase diagram of silicon to answer the following questions. For the y-axis of th ...
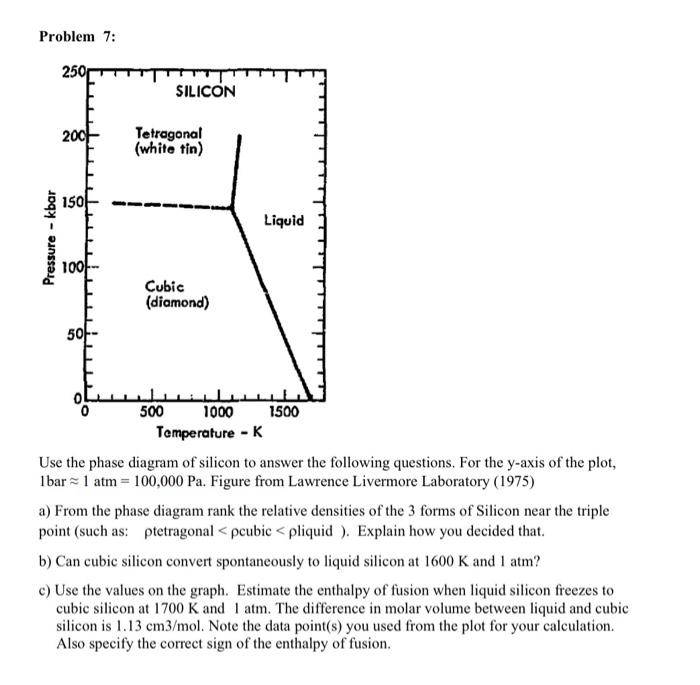
Problem 7: Use the phase diagram of silicon to answer the following questions. For the -axis of the plot, . Figure from Lawrence Livermore Laboratory (1975) a) From the phase diagram rank the relative densities of the 3 forms of Silicon near the triple point (such as: tetragonal cubic liquid ). Explain how you decided that. b) Can cubic silicon convert spontaneously to liquid silicon at and ? c) Use the values on the graph. Estimate the enthalpy of fusion when liquid silicon freezes to cubic silicon at and . The difference in molar volume between liquid and cubic silicon is . Note the data point(s) you used from the plot for your calculation. Also specify the correct sign of the enthalpy of fusion.