Home /
Expert Answers /
Chemical Engineering /
problem-01-a-binary-liquid-mixture-of-n-pentane-1-and-n-heptane-2-make-a-ideal-solution-the-va-pa518
(Solved): Problem 01: A binary liquid mixture of n-pentane (1) and n-heptane (2) make a ideal solution. The va ...
Problem 01:
A binary liquid mixture of n-pentane (1) and n-heptane (2) make a ideal solution. The vapor
pressure of the pure component are given by Antoine equation. Prepare
a) P-x-y diagram at 70 °C.
b) T-x-y diagram at 10.325 kPa pressure.
c) If the equimolar vapor mixture of this two at 90 °C is isothermally compressed till
condensation starts. Determine the pressure at which condensation begins and
composition of the liquid that form.
Components
n-pentane (1)
n-heptane (2)
A
6.87633
6.89386
B
+
1075.780
1264.370
C
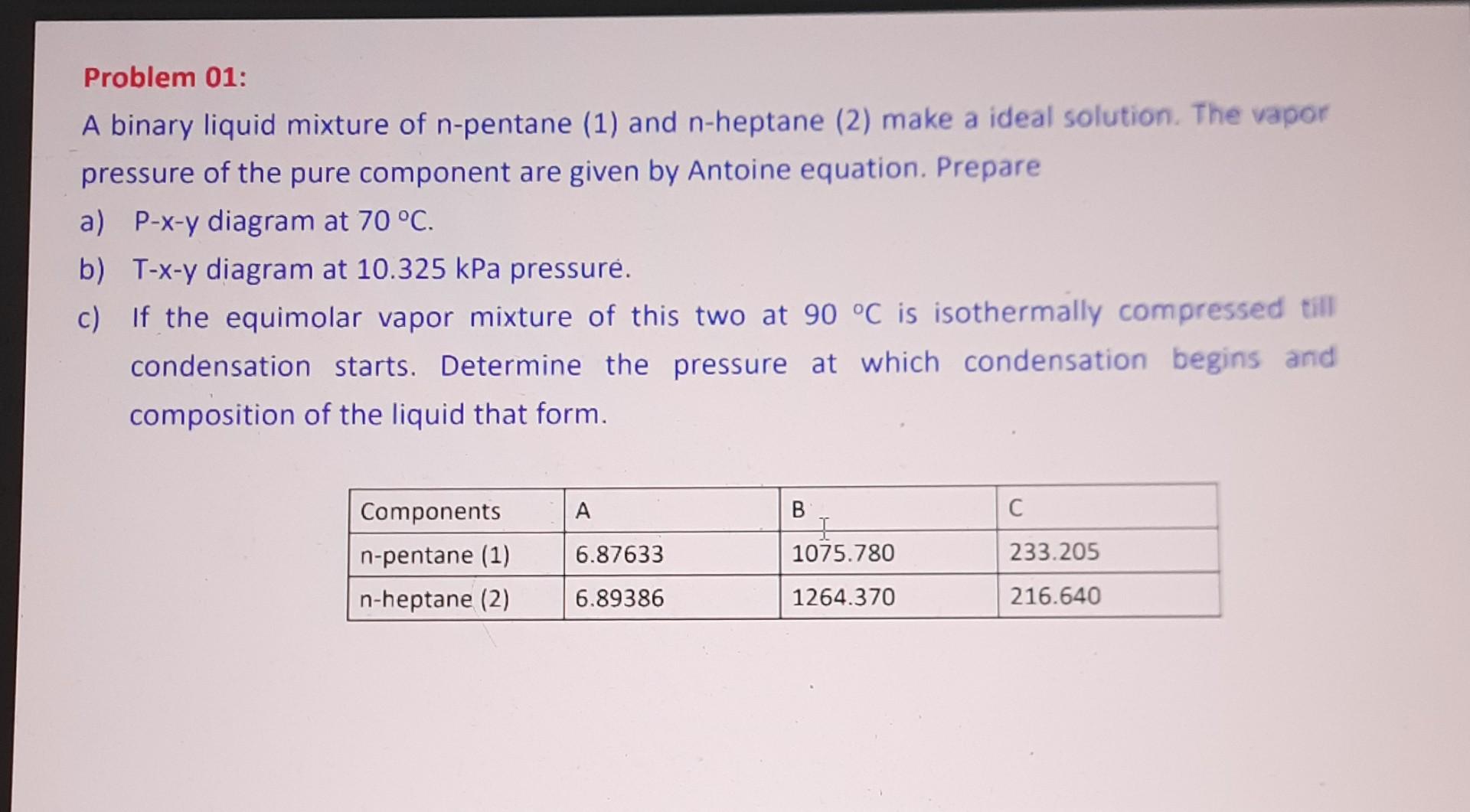
233.205
216.640
Problem 01: A binary liquid mixture of -pentane (1) and -heptane (2) make a ideal solution. The vapor pressure of the pure component are given by Antoine equation. Prepare a) -x-y diagram at . b) diagram at pressure. c) If the equimolar vapor mixture of this two at is isothermally compressed till condensation starts. Determine the pressure at which condensation begins and composition of the liquid that form.