Home /
Expert Answers /
Chemistry /
prelab-for-reaction-of-crystal-violet-and-sodium-hydroxide-show-all-work-include-correct-significa-pa465
(Solved): Prelab for Reaction of Crystal Violet and Sodium Hydroxide Show all work. Include correct significa ...

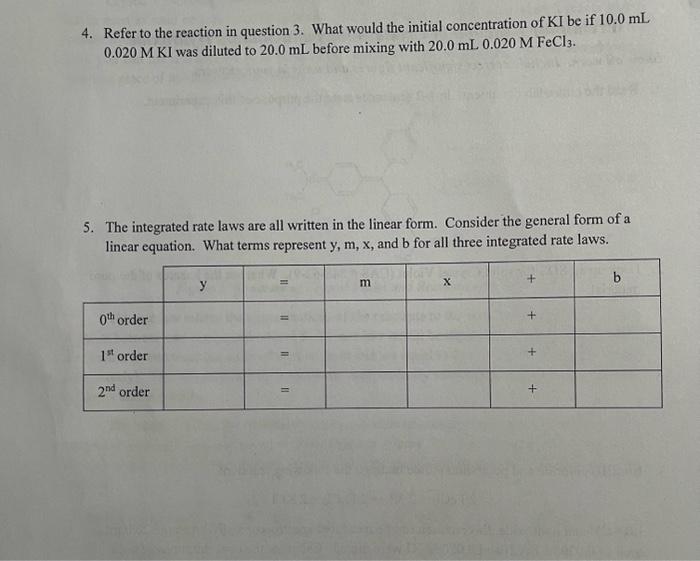
Prelab for Reaction of Crystal Violet and Sodium Hydroxide Show all work. Include correct significant figures and units in your answers. 2. Look up an SDS for solid Crystal Violet (CAS \# 548-62-9). What are the hazards of the pure material. 3. Iron(III) chloride and potassium iodide undergo the following reaction: Like CV and hydroxide, this reaction can be measured spectroscopically. Assume each of and were mixed. Calculate the concentrations of both reactants immediately after the two solutions were mixed but before any reaction occurred (this is the initial concentration of a reaction).
4. Refer to the reaction in question 3. What would the initial concentration of be if was diluted to before mixing with . 5. The integrated rate laws are all written in the linear form. Consider the general form of a linear equation. What terms represent , and for all three integrated rate laws.