Home /
Expert Answers /
Chemistry /
predict-the-equilibrium-partial-pressure-of-mathrm-hbr-in-the-reaction-described-below-by-c-pa768
(Solved): Predict the equilibrium partial pressure of \( \mathrm{HBr} \) in the reaction described below by c ...
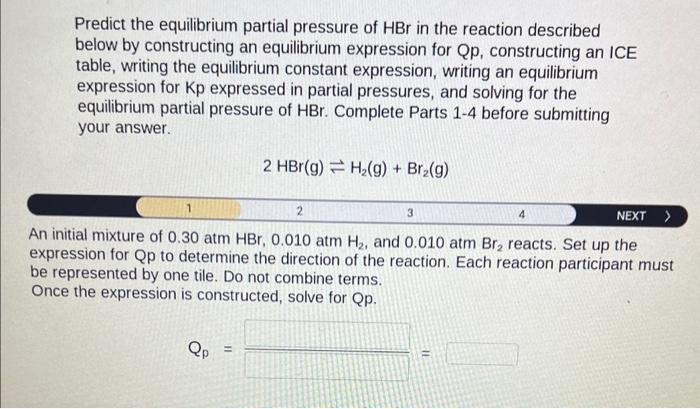
Predict the equilibrium partial pressure of \( \mathrm{HBr} \) in the reaction described below by constructing an equilibrium expression for \( \mathrm{Qp} \), constructing an ICE table, writing the equilibrium constant expression, writing an equilibrium expression for Kp expressed in partial pressures, and solving for the equilibrium partial pressure of \( \mathrm{HBr} \). Complete Parts 1-4 before submitting your answer. \[ 2 \mathrm{HBr}(\mathrm{g}) \rightleftharpoons \mathrm{H}_{2}(\mathrm{~g})+\mathrm{Br}_{2}(\mathrm{~g}) \] An initial mixture of \( 0.30 \mathrm{~atm} \mathrm{HBr}, 0.010 \mathrm{~atm} \mathrm{H}_{2} \), and \( 0.010 \mathrm{~atm} \mathrm{Br}_{2} \) reacts. Set up the expression for \( \mathrm{Qp} \) to determine the direction of the reaction. Each reaction participant must be represented by one tile. Do not combine terms. Once the expression is constructed, solve for Qp. \[ Q_{p}= \]
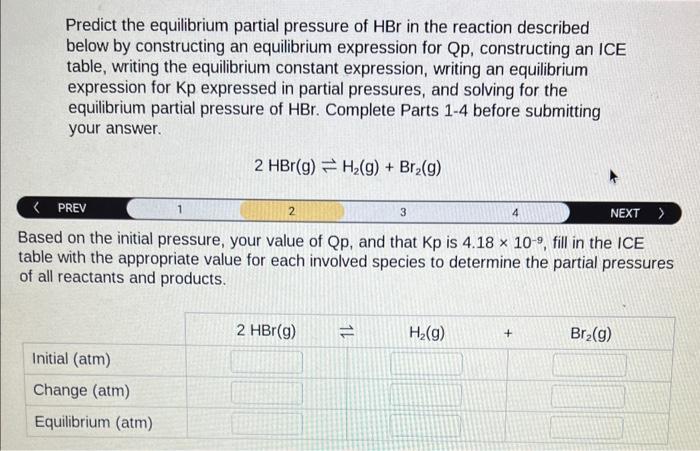
Predict the equilibrium partial pressure of \( \mathrm{HBr} \) in the reaction described below by constructing an equilibrium expression for Qp, constructing an ICE table, writing the equilibrium constant expression, writing an equilibrium expression for \( \mathrm{Kp} \) expressed in partial pressures, and solving for the equilibrium partial pressure of \( \mathrm{HBr} \). Complete Parts 1-4 before submitting your answer. \[ 2 \mathrm{HBr}(\mathrm{g}) \rightleftharpoons \mathrm{H}_{2}(\mathrm{~g})+\mathrm{Br}_{2}(\mathrm{~g}) \] PREV 1 3 NEXT > Based on the initial pressure, your value of \( \mathrm{Qp} \), and that \( \mathrm{Kp} \) is \( 4.18 \times 10^{-9} \), fill in the ICE table with the appropriate value for each involved species to determine the partial pressures of all reactants and products.
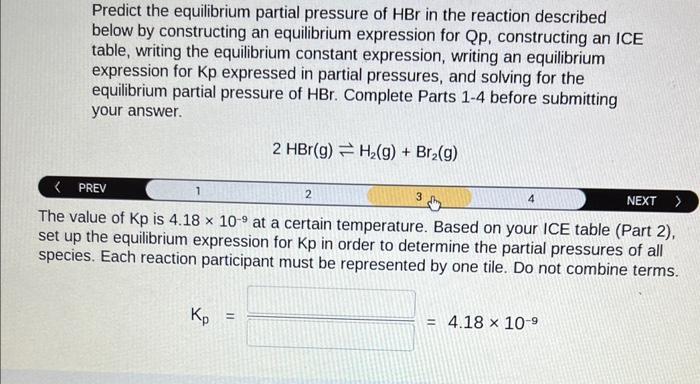
Predict the equilibrium partial pressure of \( \mathrm{HBr} \) in the reaction described below by constructing an equilibrium expression for Qp, constructing an ICE table, writing the equilibrium constant expression, writing an equilibrium expression for Kp expressed in partial pressures, and solving for the equilibrium partial pressure of \( \mathrm{HBr} \). Complete Parts 1-4 before submitting your answer. \[ 2 \mathrm{HBr}(\mathrm{g}) \rightleftharpoons \mathrm{H}_{2}(\mathrm{~g})+\mathrm{Br}_{2}(\mathrm{~g}) \] \begin{tabular}{l|cccccc} \( \langle \) PREV & 1 & 2 & 4 & NEXT \end{tabular} set up the equilibrium expression for \( \mathrm{Kp} \) in order to determine the partial pressures of all species. Each reaction participant must be represented by one tile. Do not combine terms. \[ \mathrm{K}_{\mathrm{p}}= \]
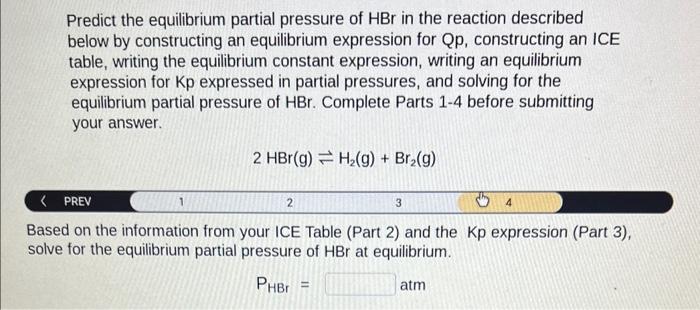
Predict the equilibrium partial pressure of \( \mathrm{HBr} \) in the reaction described below by constructing an equilibrium expression for Qp, constructing an ICE table, writing the equilibrium constant expression, writing an equilibrium expression for Kp expressed in partial pressures, and solving for the equilibrium partial pressure of \( \mathrm{HBr} \). Complete Parts 1-4 before submitting your answer. \[ 2 \mathrm{HBr}(\mathrm{g}) \rightleftharpoons \mathrm{H}_{2}(\mathrm{~g})+\mathrm{Br}_{2}(\mathrm{~g}) \] < PREV Based on the information from your ICE Table (Part 2) and the Kp expression (Part 3), solve for the equilibrium partial pressure of \( \mathrm{HBr} \) at equilibrium. \[ \mathrm{P}_{\mathrm{HBr}}=\quad \mathrm{atm} \]
Expert Answer
The given reaction is: 2HBr(g)????HA2(g)+BrA2(g) Given data is: PHBr=0.30atmPH2=0.010atmPBr2=0.010atm?QP=PH2×PBr2(PHBr)2=0.010×0.010(0.30)2QP=0.00111