Home /
Expert Answers /
Chemistry /
predict-the-equilibrium-concentration-of-ca-in-the-reaction-described-below-by-constructing-an-pa766
(Solved): Predict the equilibrium concentration of Ca+ in the reaction described below by constructing an ...
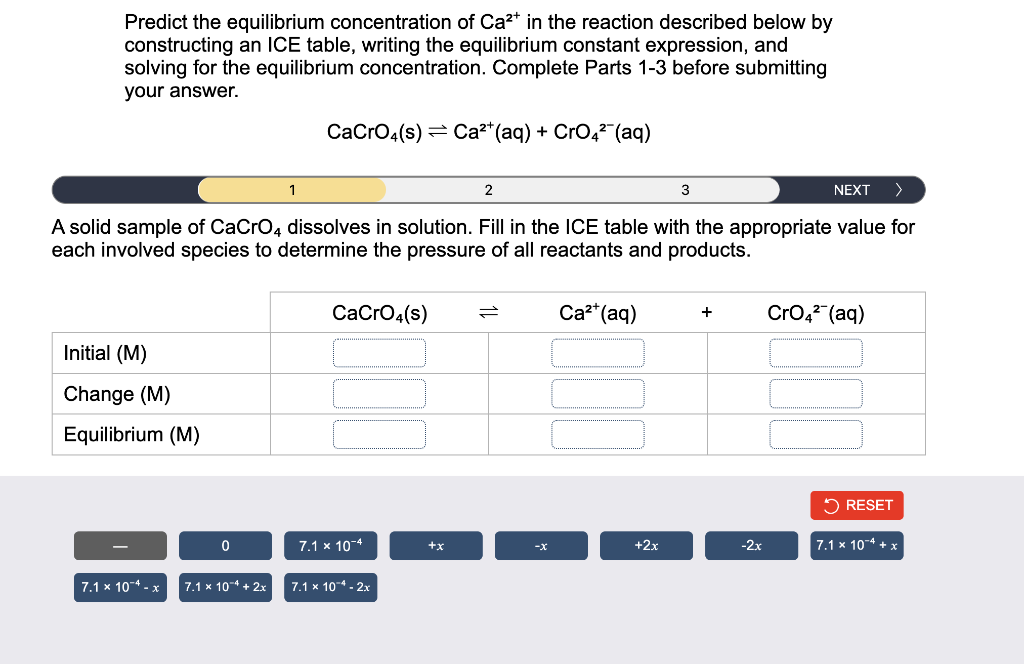
Predict the equilibrium concentration of Ca²+ in the reaction described below by constructing an ICE table, writing the equilibrium constant expression, and solving for the equilibrium concentration. Complete Parts 1-3 before submitting your answer. CaCrO4(s) Ca²+ (aq) + CrO4² (aq) 1 2 3 NEXT > A solid sample of CaCrO4 dissolves in solution. Fill in the ICE table with the appropriate value for each involved species to determine the pressure of all reactants and products. CaCrO4(s) = Ca²+ (aq) + CrO?² (aq) Initial (M) Change (M) Equilibrium (M) +x 7.1 x 10-4-x 0 7.1 x 104 + 2x 7.1 x 10-4 7.1 x 104 - 2x -X +2x -2x RESET 7.1 x 104 + x
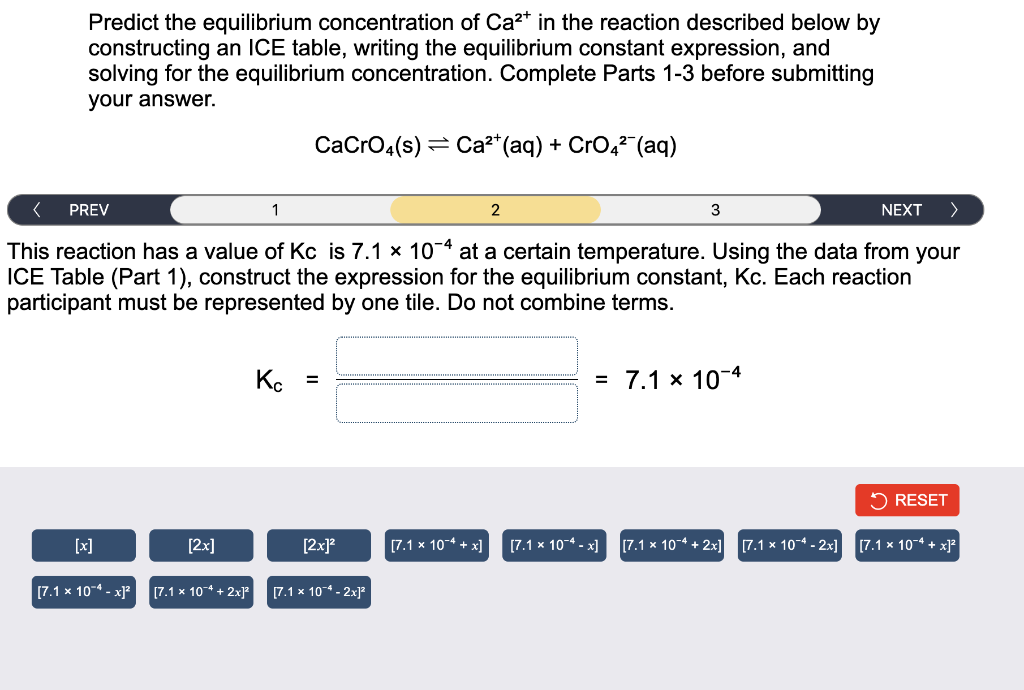
Predict the equilibrium concentration of Ca²* in the reaction described below by constructing an ICE table, writing the equilibrium constant expression, and solving for the equilibrium concentration. Complete Parts 1-3 before submitting your answer. CaCrO4(s) Ca²+ (aq) + CrO4²¯ (aq) < PREV 1 2 3 NEXT > This reaction has a value of Kc is 7.1 x 104 at a certain temperature. Using the data from your ICE Table (Part 1), construct the expression for the equilibrium constant, Kc. Each reaction participant must be represented by one tile. Do not combine terms. Kc = = 7.1 x 10-4 RESET [x] [2x] [7.1 x 10-4 + x] [7.1 x 10-4-x] [7.1 x 104 + 2x] [7.1 x 10-4-2x] [7.1 x 10-4+x]² [7.1 x 10-4-x]² [7.1 × 10 + 2x]² [2x]² [7.1 x 104 - 2x]²
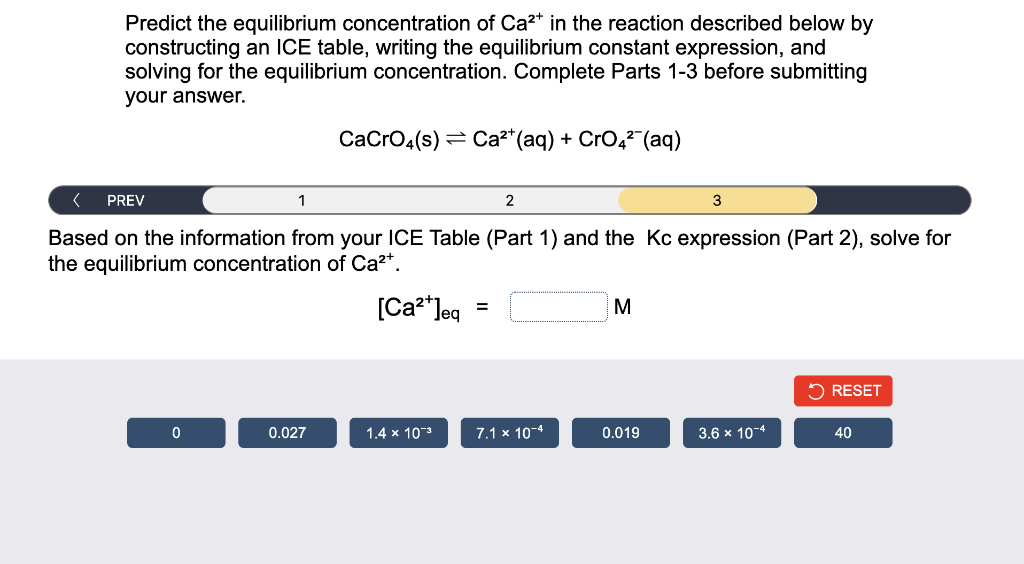
Predict the equilibrium concentration of Ca²+ in the reaction described below by constructing an ICE table, writing the equilibrium constant expression, and solving for the equilibrium concentration. Complete Parts 1-3 before submitting your answer. CaCrO4(s) = Ca²+ (aq) + CrO4² (aq) < PREV 1 2 3 Based on the information from your ICE Table (Part 1) and the Kc expression (Part 2), solve for the equilibrium concentration of Ca²+. [Ca²+]eq = M RESET 0 0.027 0.019 3.6 × 10-4 40 1.4 x 10³ 7.1 x 10-4
Expert Answer
Answer:- This question is answered by u