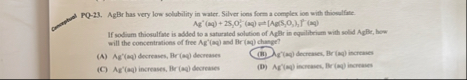Home /
Expert Answers /
Chemistry /
pq-23-agbr-has-very-low-solubility-in-water-silver-ions-form-a-complex-ioe-with-thiomelfive-ag-39-pa823
(Solved): PQ-23. AgBr has very low solubility in water. Silver ions form a complex ioe with thiomelfive: Ag^(' ...
PQ-23. AgBr has very low solubility in water. Silver ions form a complex ioe with thiomelfive:
Ag^(')(aq) 2(S),O,-(mq)?[Aq,S_(3),O,)_(3)If sodium thionulfate is added to a saturated solution of Ap flir in equilitriam with sold Ag Ec , how will the concentrations of free
Ag^( )(aq)and
Br(mq)change? (A)
Ag^(')(mq)decreases,
Br(aq)decreases (ii)
\lambda _(g)(ay)decrenies,
Br(mq)incrowes (C) Afr'(aq) increases, Mr (aq) decreases (i9)
Ar^(')(m)incrowec,
Br(m)ingrawes