Home /
Expert Answers /
Chemistry /
pm-using-the-equilibrium-constant-the-reversible-chemical-reaction-mathrm-a-a-q-mathr-pa143
(Solved): \( \pm \) Using the Equilibrium Constant The reversible chemical reaction \[ \mathrm{A}(a q)+\mathr ...
![What is the final concentration of \( \mathrm{D} \) at equilibrium if the initial concentrations are \( [\mathrm{A}]=1.00 \ma](https://media.cheggcdn.com/study/5e7/5e7e0f11-8a9c-4daf-95ea-5a94f53f66ef/image)
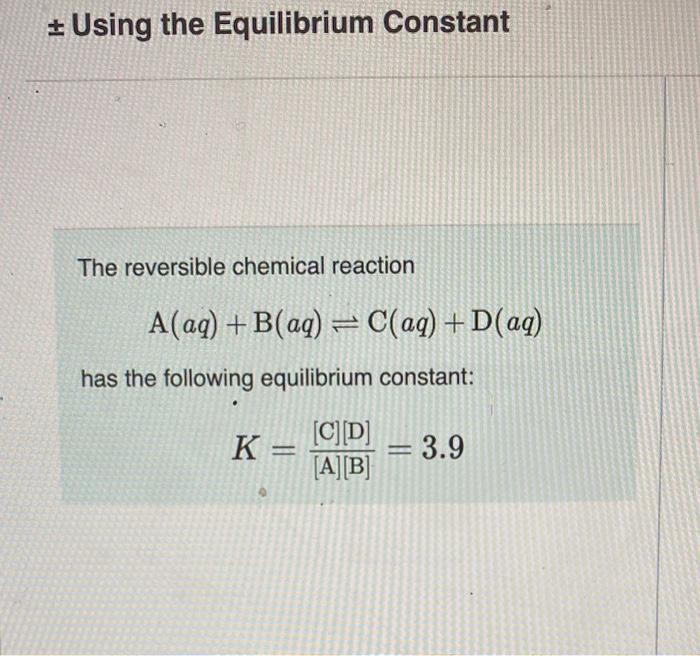
\( \pm \) Using the Equilibrium Constant The reversible chemical reaction \[ \mathrm{A}(a q)+\mathrm{B}(a q) \rightleftharpoons \mathrm{C}(a q)+\mathrm{D}(a q) \] has the following equilibrium constant: \[ K=\frac{[\mathrm{C}][\mathrm{D}]}{[\mathrm{A}][\mathrm{B}]}=3.9 \]
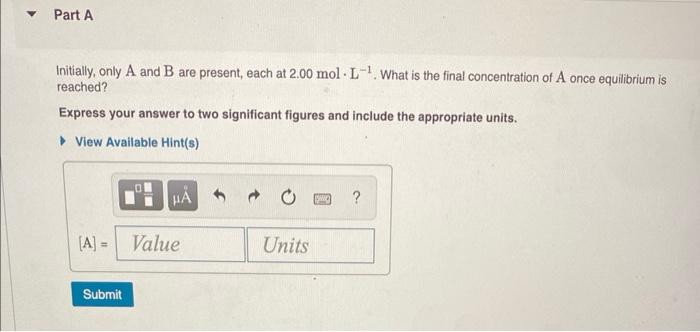
Initially, only \( \mathrm{A} \) and \( \mathrm{B} \) are present, each at \( 2.00 \mathrm{~mol} \cdot \mathrm{L}^{-1} \). What is the final concentration of \( \mathrm{A} \) once equilibrium is reached? Express your answer to two significant figures and include the appropriate units.
What is the final concentration of \( \mathrm{D} \) at equilibrium if the initial concentrations are \( [\mathrm{A}]=1.00 \mathrm{~mol} \cdot \mathrm{L}^{-1} \) and \( [\mathrm{B}]=2.00 \mathrm{~mol} \cdot \mathrm{L}^{-1} \) ? Express your answer to two significant figures and include the appropriate units.