Home /
Expert Answers /
Chemistry /
pls-help-model-3-some-common-acids-and-bases-acids-dissociate-in-water-to-give-hydrogen-left-pa295
(Solved): pls help Model 3 : Some common acids and bases Acids dissociate in water to give hydrogen \( \left(\ ...
pls help
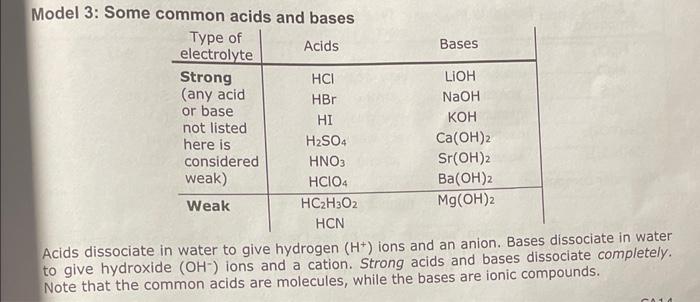




Model 3 : Some common acids and bases Acids dissociate in water to give hydrogen \( \left(\mathrm{H}^{+}\right) \)ions and an anion. Bases dissociate in water to give hydroxide \( \left(\mathrm{OH}^{-}\right) \)ions and a cation. Strong acids and bases dissociate completely. Note that the common acids are molecules, while the bases are ionic compounds.
Critical Thinking Questions: Manager: for CTQs 3-7, identify a different team member to give the first explanathon 3 . Consider Modei 3. What do all the molecular formulos of the acids have in commonty 3. Consider Model 3. What do all the molecular formulas of the acids have in common? 4. How can you recognize an acid from the molecular formula? 5. How can you recognize a base from its formula? 6. What happens to strong acids when dissolved in water? 7. What happens to weak acids when dissolved in water? Discuss with your team and prepare to share the team answer with the class. 8. Are all acids strong electrolytes? Discuss with your team and write the team's explanation. Record your team's answer (on the report form, if applicable). 9. Describe what happens to the ions in solid sodium hydroxide \( (\mathrm{NaOH}) \) during the proce of dissolving in water. 10. Water is, in actuality, an extremely weak acid (see CTQ 4). Explain, then, how water often considered to be a nonelectrolyte. (You may wish to refer to Models 1 and 2). 11. Comment on strengths and needed improvements for your teamwork so far. Model 4: Solubilities of some ionic compounds Molecular compounds (other than acids) do not dissociate significantly in water and 50 are nonelectrolytes. The phase label (aq), meaning "aqueous," can be used to show that a species is dissolved in water. We will not consider how to predict if an ionic compound is soluble in water until later in the course.
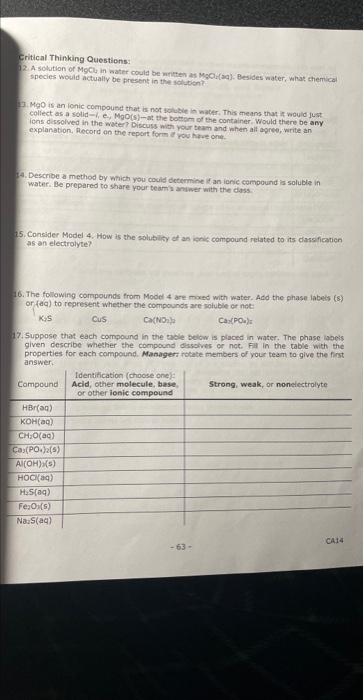
Critical Thinking Questions: 22. A solvtion of MgClu in water could be mriter as MgCulaq). Besides water, whas themical species would actually be present in the solutien?? 2. MgO is an ionic compoued that is not solukie in water. This means that in would just collect as a solid \( -1 \), e., MoO(s) - at the botsom of the container. Would there be any icns disolved in the water Discuss with your teat and when ali agree, write an explanation, flecord an the report form af you hife one. 4. Describe a method by which you couid dectrmine if an ienic compound is soluble in water. Be prepared to share your team a andwer with the diass. 75. Coneider Model 4. How is the solubisy ef an ishic compound reiated to its dassficatien as an electrolyte? 16. The following corrpounds from Model 4 are mosed with water. Add the phase labeis (8) or (aq) to represent whether the compounds are spiuble or not- Kis Cus Cavions \( \mathrm{Ca},(\mathrm{POa}) \mathrm{\pi} \) 7. Suppose that each compound in the table below is placed in water. The phase iaseis given describe whether the compound dassolves or nct. Fil in the table with the properties for each compound. Managern rotate members of yout team to-give the first answert
Expert Answer
Answer (3) - All the molecular formula of the acids given in the model 3 have one common hydrogen atom (which is written very first in each molecular formula). Answer (4) - The molecular fo