Home /
Expert Answers /
Chemistry /
please-trying-figureout-the-theoritical-yield-help-prelab-solventless-aldol-condensation-of-1-inda-pa179
(Solved): please trying figureout the theoritical yield. help Prelab: Solventless Aldol Condensation of 1-Inda ...
please trying figureout the theoritical yield. help

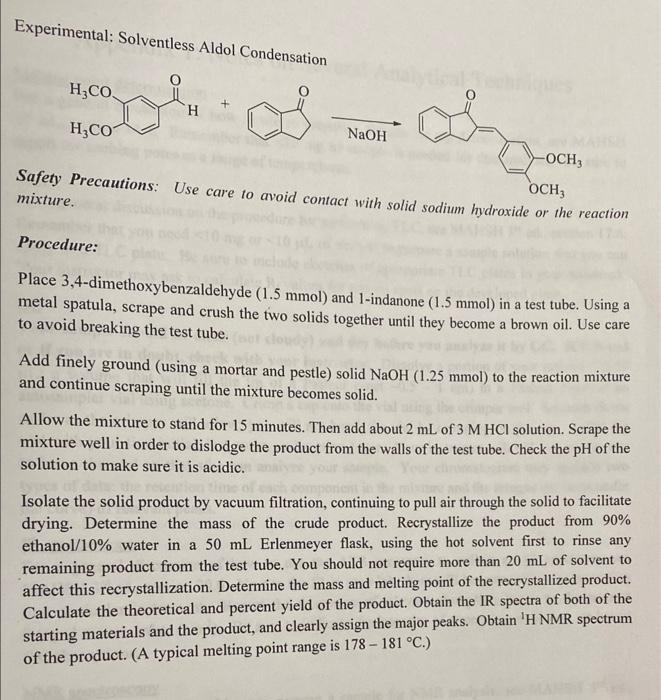
Prelab: Solventless Aldol Condensation of 1-Indanone solute in the solution. Structure of aldol condensation product: \( \Rightarrow B-H y d r o x y \) aldehyde. Limiting reagent: 3,4 almetcoxy Bentaldende Theoretical yield:
Experimental: Solventless Aldol Condensation \( \underset{\mathrm{NaOH}}{\longrightarrow} \) Safety Precautions: Use care to avoid contact with solid sodium hydroxide or the reaction mixture. Procedure: Place 3,4-dimethoxybenzaldehyde \( (1.5 \mathrm{mmol}) \) and 1-indanone \( (1.5 \mathrm{mmol}) \) in a test tube. Using a metal spatula, scrape and crush the two solids together until they become a brown oil. Use care to avoid breaking the test tube. Add finely ground (using a mortar and pestle) solid \( \mathrm{NaOH}(1.25 \mathrm{mmol}) \) to the reaction mixture and continue scraping until the mixture becomes solid. Allow the mixture to stand for 15 minutes. Then add about \( 2 \mathrm{~mL} \) of \( 3 \mathrm{M} \mathrm{HCl} \) solution. Scrape the mixture well in order to dislodge the product from the walls of the test tube. Check the \( \mathrm{pH} \) of the solution to make sure it is acidic. Isolate the solid product by vacuum filtration, continuing to pull air through the solid to facilitate drying. Determine the mass of the crude product. Recrystallize the product from \( 90 \% \) ethanol/10\% water in a \( 50 \mathrm{~mL} \) Erlenmeyer flask, using the hot solvent first to rinse any remaining product from the test tube. You should not require more than \( 20 \mathrm{~mL} \) of solvent to affect this recrystallization. Determine the mass and melting point of the recrystallized product. Calculate the theoretical and percent yield of the product. Obtain the IR spectra of both of the starting materials and the product, and clearly assign the major peaks. Obtain \( { }^{1} \mathrm{H} \) NMR spectrum of the product. (A typical melting point range is \( 178-181^{\circ} \mathrm{C} \).)
Expert Answer
Solution: The limiting reagent is the reactant which gets consumed first in the reaction. Here, mole ratio betw