Home /
Expert Answers /
Chemistry /
please-please-fill-out-entirely-and-clear-b-write-the-correctly-balanced-equation-and-the-type-of-pa948
(Solved): PLEASE PLEASE fill out entirely and clear!! B. Write the correctly balanced equation and the type of ...
PLEASE PLEASE fill out entirely and clear!!



B. Write the correctly balanced equation and the type of reaction 1. Zinc reacts with sulfur to produce zinc(IV) sulfide. 3. Silver nitrate reacts with nickel to produce rickel(ii) nitrate and silver. 4. Aluminum reacts with iron (iii) oxide to form aluminum oxide and iron. 5. Magnesium nitride is heated untit it forms magnesium metal and nitrogen gas. 7. Iron (ii) sulfate reacts with ammonium sufide to form iron (ii) sulfide and ammonium sulfate. 8. Nickel (iI) chlorate breaks apart to become nickel (ii) chloride and oxygen. C. Net Ionic Equations - Write the complete ionic equation for the following reactions and, then, reduce the equation by eliminating the spectator ions to create the net ionic equation. Ionic Equation: Net lonic Equation: 2. Aqueous potassium sulfide reacts with a solution of cadmium chloride to form solid cadmium sultide and aqueous potassium chloride. Balanced Equation: Ionic Equation: Net lonic Equation:
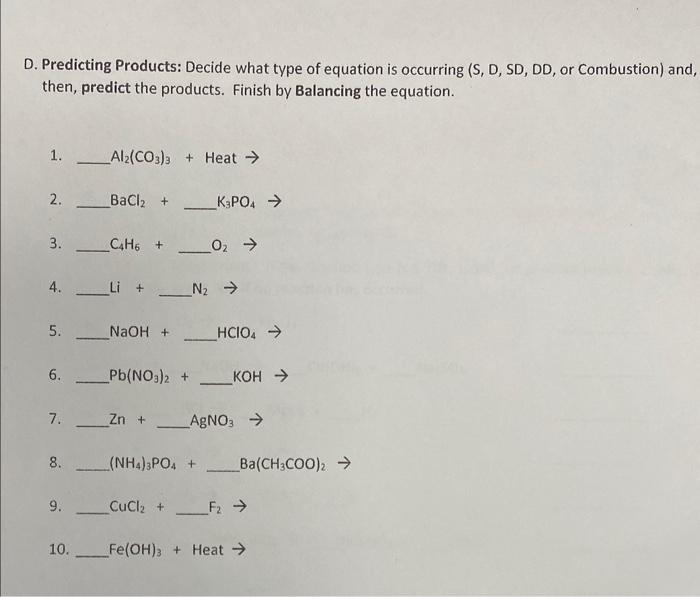
D. Predicting Products: Decide what type of equation is occurring (S, D, SD, DD, or Combustion) and, then, predict the products. Finish by Balancing the equation. 1. \( \mathrm{Al}_{2}\left(\mathrm{CO}_{3}\right)_{3}+\mathrm{Heat} \rightarrow \) 2. \( \mathrm{BaCl}_{2}+\mathrm{K}_{3} \mathrm{PO}_{4} \rightarrow \) 3. \( \mathrm{C}_{4} \mathrm{H}_{6}+\mathrm{O}_{2} \rightarrow \) 4. \( -\mathrm{Li}+\mathrm{N}_{2} \rightarrow \) 5. \( \mathrm{NaOH}+\underset{\mathrm{HClO}_{4} \rightarrow}{ } \rightarrow \) 8. \( \left(\mathrm{NH}_{4}\right)_{3} \mathrm{PO}_{4}+\ldots\left(\mathrm{CH}_{3} \mathrm{COO}\right)_{2} \rightarrow \) 9. \( \mathrm{CuCl}_{2}+\mathrm{F}_{2} \rightarrow \) 10. \( \mathrm{Fe}(\mathrm{OH})_{3}+ \) Heat \( \rightarrow \)
Expert Answer
1. Zn + 2S -------> ZnS2 synthesis or combination reaction 3. 2AgNO3 + Ni -----> Ni(NO3)2 + 2Ag-------------- single displancement reaction 4. Al + Fe2O3 -----> Al2O3 + Fe ----------------- single displancement reaction 5. 2Mg2N3--------> 4Mg +