Home /
Expert Answers /
Chemistry /
please-help-sodium-iodide-reacts-with-alkyl-halides-in-acetone-to-give-sodium-chloride-and-iodi-pa911
(Solved): please help! - Sodium iodide reacts with alkyl halides in acetone to give sodium chloride and - Iodi ...
please help!
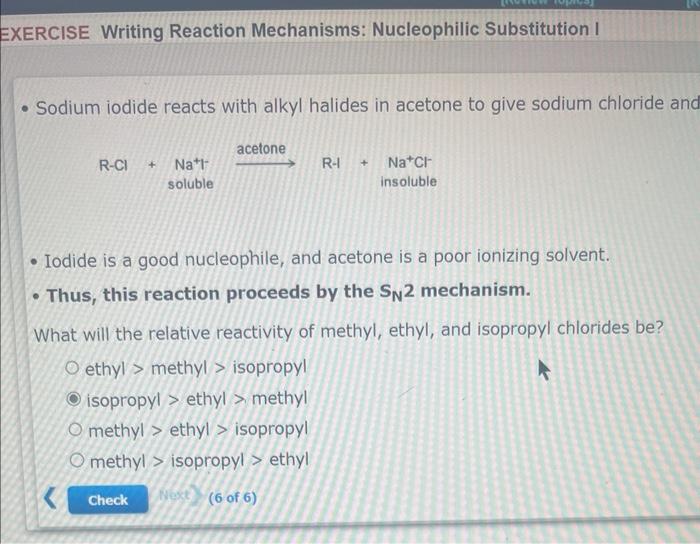

- Sodium iodide reacts with alkyl halides in acetone to give sodium chloride and - Iodide is a good nucleophile, and acetone is a poor ionizing solvent. - Thus, this reaction proceeds by the \( S_{N} 2 \) mechanism. What will the relative reactivity of methyl, ethyl, and isopropyl chlorides be? ethyl \( > \) methyl \( > \) isopropyl isopropyl \( > \) ethyl \( > \) methyl methyl \( > \) ethyl \( > \) isopropyl methyl \( > \) isopropyl \( > \) ethyl