Home /
Expert Answers /
Chemistry /
please-help-order-and-rate-law-of-a-reaction-the-overall-order-of-an-elementary-step-directly-co-pa288
(Solved): Please Help!!!! Order and rate law of a reaction The overall order of an elementary step directly co ...
Please Help!!!!
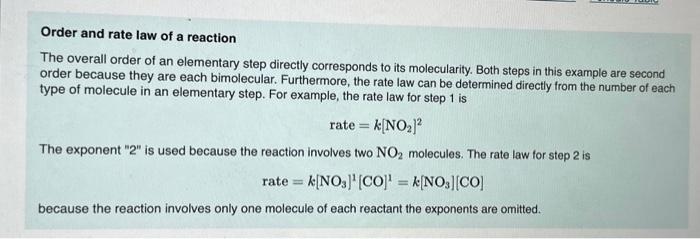

Order and rate law of a reaction The overall order of an elementary step directly corresponds to its molecularity. Both steps in this example are second order because they are each bimolecular. Furthermore, the rate law can be determined directly from the number of each type of molecule in an elementary step. For example, the rate law for step 1 is The exponent "2" is used because the reaction involves two molecules. The rate law for step 2 is because the reaction involves only one molecule of each reactant the exponents are omitted.
What is the overall reaction? Express your answer as a chemical equation. View Available Hint(s) A chemical reaction does not occur for this question.
What is the rate law for step 1 of this reaction? Express your answer in standard MasteringChemistry notation. For example, if the rate law is type . View Available Hint(s) Rate
What is the rate law for step 2 of this reaction? Express your answer in standard MasteringChemistry notation. For example, if the rate law is type . View Available Hint(s) Rate