Home /
Expert Answers /
Nursing /
please-help-me-to-answer-this-drug-fisage-calculations-3-amp-4-label-3-new-ndc-new-product-app-pa977
(Solved): please help me to answer this? (drug fisage calculations) 3 & 4 Label #3 New NDC New Product App ...
please help me to answer this? (drug fisage calculations) 3 & 4
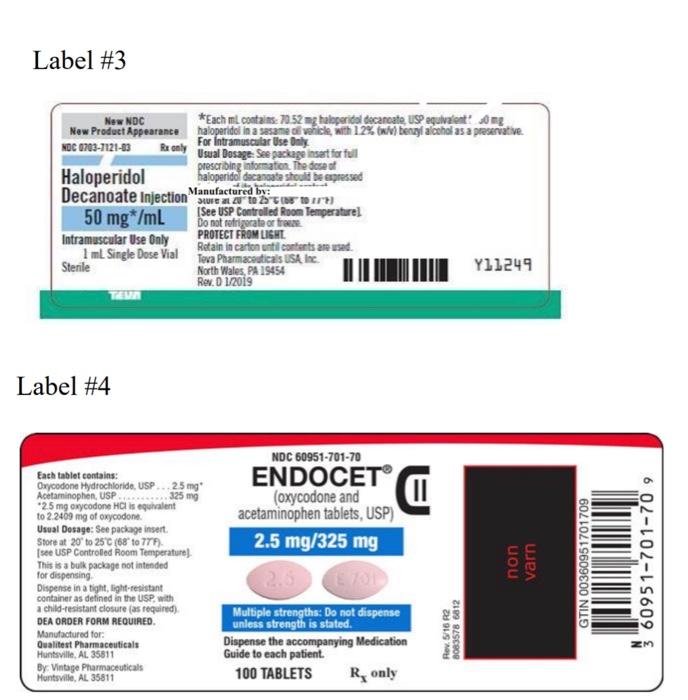



Label #3 New NDC New Product Appearance NOC 0703-7121-03 R only Haloperidol Decanoate Injection 50 mg*/mL Intramuscular Use Only 1 ml Single Dose Vial Sterile TAVA Label #4 Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required) DEA ORDER FORM REQUIRED. Manufactured for: Qualitest Pharmaceuticals Huntsville, AL 35811 *Each ml contains: 70.52 mg haloperidol decanoate, USP equivalent 0 mg haloperidol in a sesame oi venicle, with 1.2% (w/v) benzyl alcohol as a preservative For Intramuscular Use Only Usual Dosage: See package insert for full prescribing information. The dose of haloperidol decanoate should be expressed kalumanidad cond By: Vintage Pharmaceuticals Huntsville, AL 35811 by: sure at 20 to 25 to 117) [See USP Controlled Room Temperature) Do not refrigerate or fra PROTECT FROM LIGHT Each tablet contains: Oxycodone Hydrochloride, USP... 2.5 mg" Acetaminophen, USP 325 mg *2.5 mg oxycodone HCI is equivalent to 2.2409 mg of oxycodone Usual Dosage: See package insert. Store at 20 to 25°C (68° to 77F). [see USP Controlled Room Temperature) This is a bulk package not intended for dispensing Retain in carton until contents are used. Teva Pharmaceuticals USA, Inc. North Wales, PA 19454 Rev. D 1/2019 NDC 60951-701-70 (oxycodone and acetaminophen tablets, USP) 2.5 mg/325 mg 2.5 TE 701 Multiple strengths: Do not dispense unless strength is stated. Dispense the accompanying Medication Guide to each patient. 100 TABLETS R only Rev. 5/16 R2 8083578 6812 Y11249 non varn GTIN 00360951701709 60951-701-70 9
Label #3 21. Trade name 23. Total volume or amount 24. Form 26. Det 27. Controlled schedule 28. Storage information 29. Madacturer's name 30. Does it have a black bos warning? Label #4 31. Trade name 32. C. 33. Total volume of ou 35. Rod 37. Controlled sunce schedule 38. Storage information
Expert Answer
The required information has been filled in the above chart from the label. Explanation for fe