Home /
Expert Answers /
Advanced Physics /
please-help-finish-chart-please-help-finish-lab-chart-part-1-a-block-of-an-unknown-metal-pa639
(Solved): please help finish chart!!!! please help finish lab chart. !! PART 1: A block of an unknown metal ...
please help finish chart!!!!
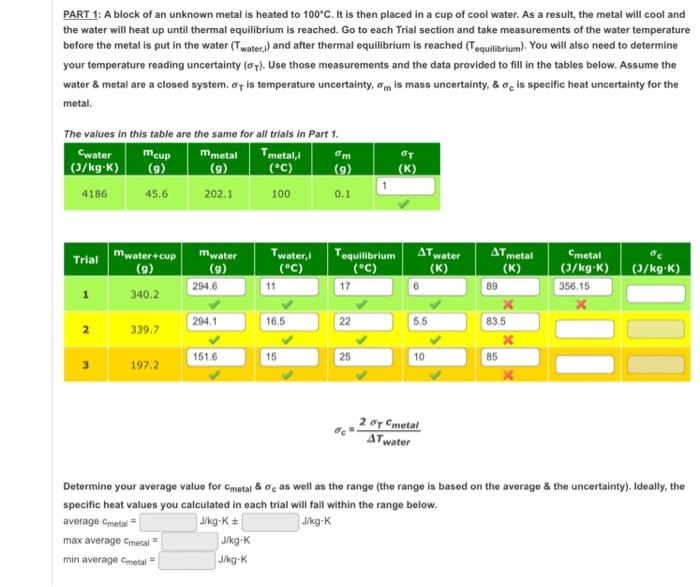

please help finish lab chart. !!
PART 1: A block of an unknown metal is heated to . It is then placed in a cup of cool water. As a result, the metal will cool and the water will heat up until thermal equilibrium is reached. Go to each Trial section and take measurements of the water temperature before the metal is put in the water and after thermal equilibrium is reached . You will also need to determine your temperature reading uncertainty . Use those measurements and the data provided to fill in the tables below. Assume the water metal are a closed system. is temperature uncertainty, is mass uncertainty, is specific heat uncertainty for the metal. The values in this table are the same for all trials in Part 1. Determine your average value for as well as the range (the range is based on the average \& the uncertainty), Ideally, the specific heat values you calculated in each trial will fall within the range below.