Home /
Expert Answers /
Chemistry /
please-help-and-put-into-the-format-so-i-can-do-the-problem-calculate-the-needed-amount-of-reac-pa368
(Solved): Please help, and put into the format so I can do the problem! Calculate the needed amount of reac ...
Please help, and put into the format so I can do the problem!
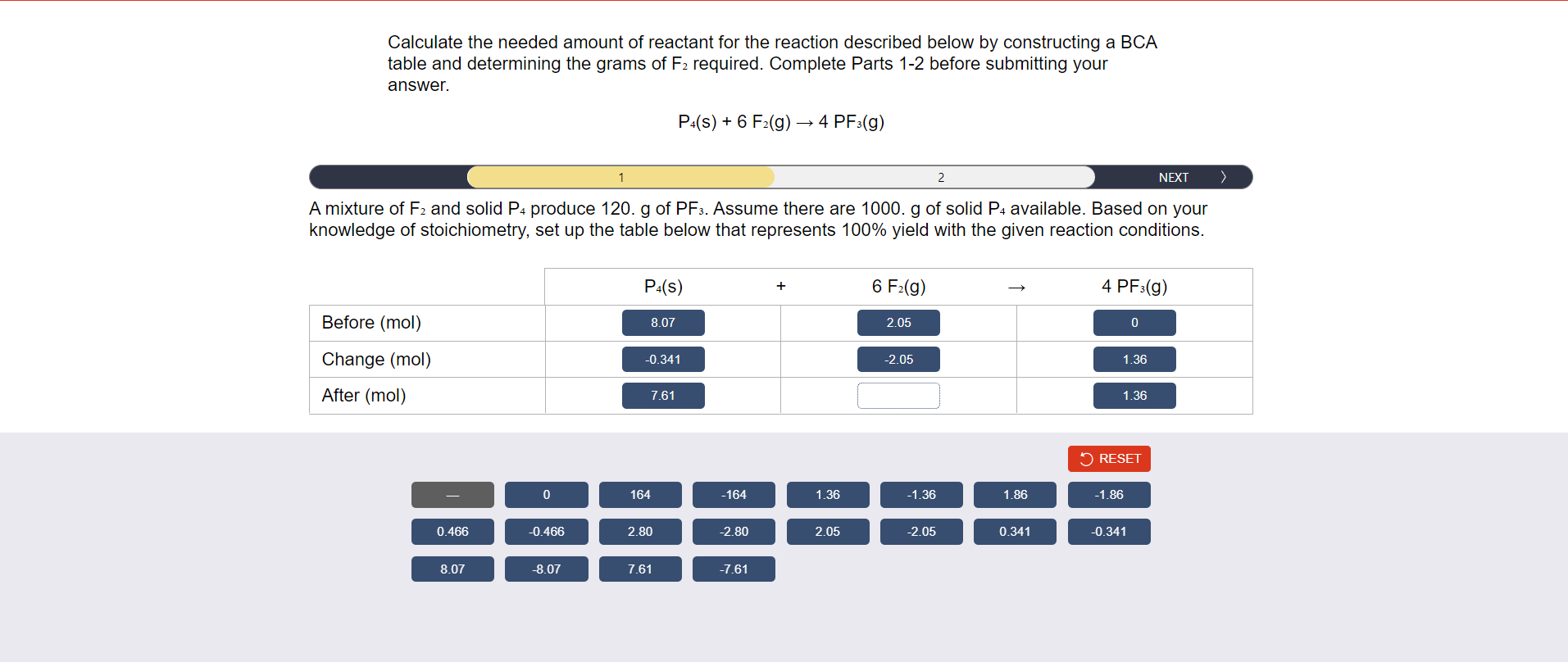
Calculate the needed amount of reactant for the reaction described below by constructing a BCA table and determining the grams of \( F_{2} \) required. Complete Parts 1-2 before submitting your answer. \[ \mathrm{P}_{4}(\mathrm{~s})+6 \mathrm{~F}_{2}(\mathrm{~g}) \rightarrow 4 \mathrm{PF}_{3}(\mathrm{~g}) \] A mixture of \( F_{2} \) and solid \( P_{4} \) produce 120. \( g \) of \( P F_{3} \). Assume there are 1000. g of solid \( P_{4} \) available. Based on your knowledge of stoichiometry, set up the table below that represents \( 100 \% \) yield with the given reaction conditions.
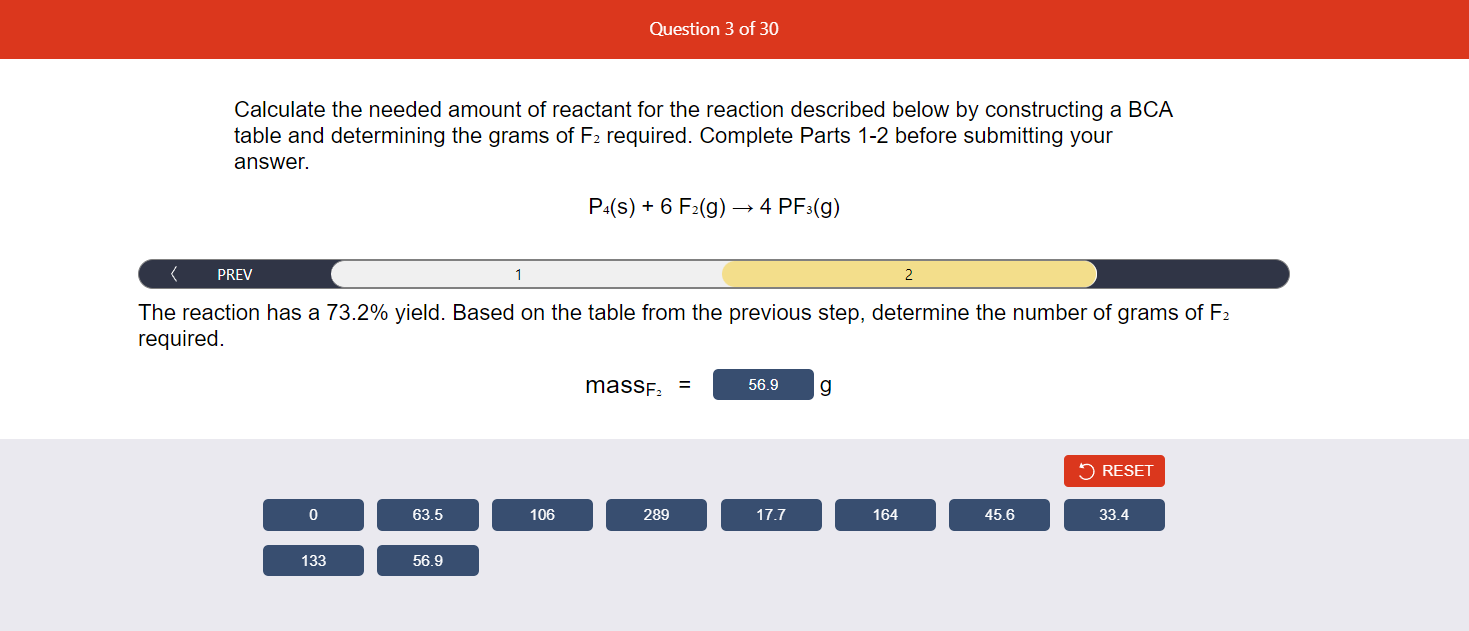
Calculate the needed amount of reactant for the reaction described below by constructing a BCA table and determining the grams of \( F_{2} \) required. Complete Parts 1-2 before submitting your answer. \[ \mathrm{P}_{4}(\mathrm{~s})+6 \mathrm{~F}_{2}(\mathrm{~g}) \rightarrow 4 \mathrm{PF}_{3}(\mathrm{~g}) \] The reaction has a \( 73.2 \% \) yield. Based on the table from the previous step, determine the number of grams of \( F_{2} \) required.