Home /
Expert Answers /
Chemistry /
please-answer-asap-according-to-the-quantum-mechanical-model-for-the-hydrogen-atom-which-of-t-pa748
(Solved): please answer asap According to the quantum-mechanical model for the hydrogen atom, which of t ...
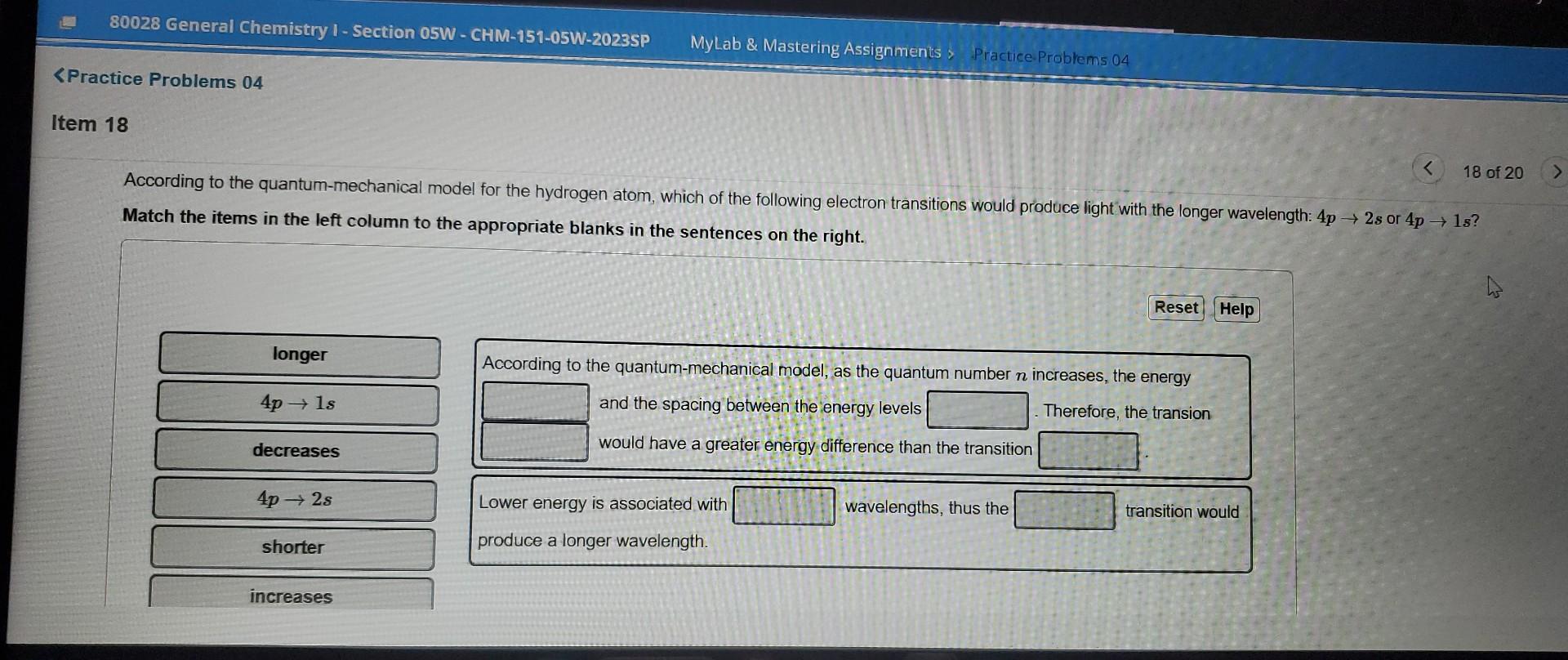
please answer asap
According to the quantum-mechanical model for the hydrogen atom, which of the following electron transitions would produce light with the longer wavelength: or Match the items in the left column to the appropriate blanks in the sentences on the right. According to the quantum-mechanical model, as the quantum number increases, the energy and the spacing between the energy levels Therefore, the transion would have a greater energy difference than the transition Lower energy is associated with wavelengths, thus the produce a longer wavelength.
Expert Answer
Answer.According to the quantum mechanical model the hydrogen atom the ener