Home /
Expert Answers /
Chemistry /
please-answer-all-thank-you-nbsp-it-is-difficult-to-prepare-an-amide-from-a-carboxylic-acid-and-an-pa714
(Solved): please answer all thank you It is difficult to prepare an amide from a carboxylic acid and an ...
please answer all thank you
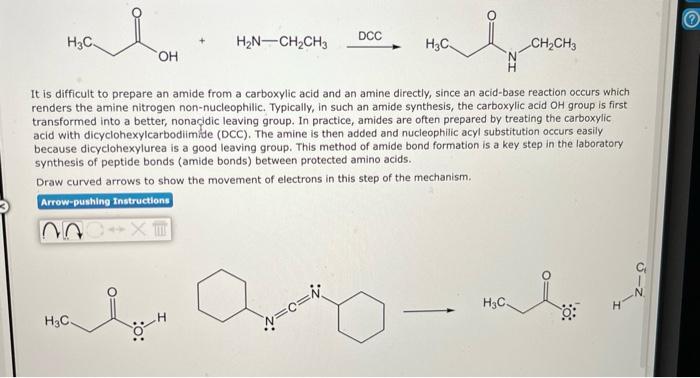







It is difficult to prepare an amide from a carboxylic acid and an amine directly, since an acid-base reaction occurs which renders the amine nitrogen non-nucleophilic. Typically, in such an amide synthesis, the carboxylic acid OH group is first transformed into a better, nonacidic leaving group. In practice, amides are often prepared by treating the carboxylic acid with dicyclohexylcarbodiimide (DCC). The amine is then added and nucleophilic acyl substitution occurs easily because dicyclohexylurea is a good leaving group. This method of amide bond formation is a key step in the laboratory synthesis of peptide bonds (amide bonds) between protected amino acids. Draw curved arrows to show the movement of electrons in this step of the mechanism.
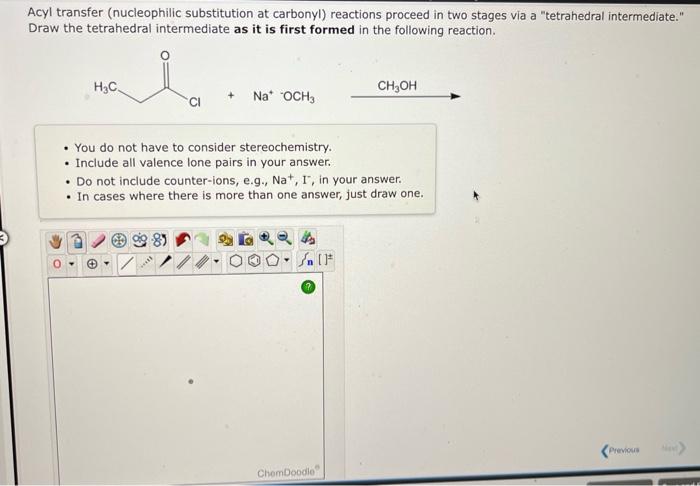
Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. - You do not have to consider stereochemistry. - Include all valence lone pairs in your answer. - Do not include counter-ions, e.g., \( \mathrm{Na}^{+}, \mathrm{I}^{-} \), in your answer. - In cases where there is more than one answer, just draw one.
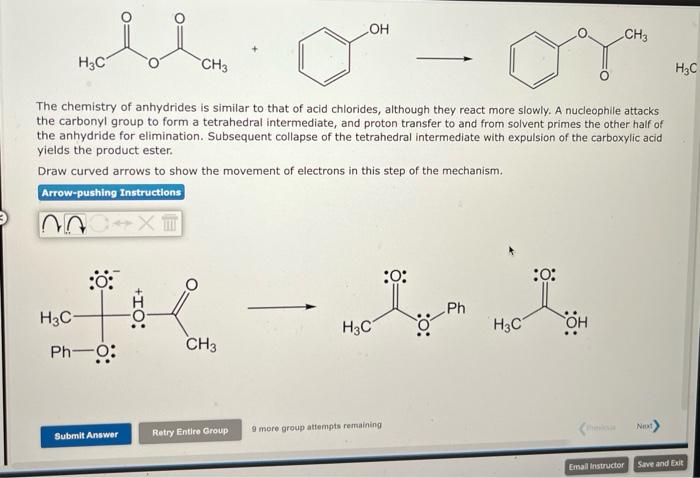
The chemistry of anhydrides is similar to that of acid chlorides, although they react more slowly. A nucleophile attacks the carbonyl group to form a tetrahedral intermediate, and proton transfer to and from solvent primes the other half of the anhydride for elimination. Subsequent collapse of the tetrahedral intermediate with expulsion of the carboxylic acid yields the product ester. Draw curved arrows to show the movement of electrons in this step of the mechanism.
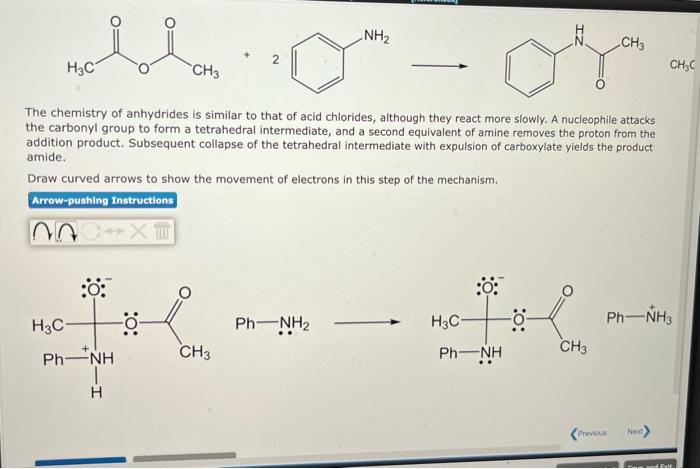
The chemistry of anhydrides is similar to that of acid chlorides, although they react more slowly. A nucleophile attacks the carbonyl group to form a tetrahedral intermediate, and a second equivalent of amine removes the proton from the addition product. Subsequent collapse of the tetrahedral intermediate with expulsion of carboxylate yields the product amide. Draw curved arrows to show the movement of electrons in this step of the mechanism.
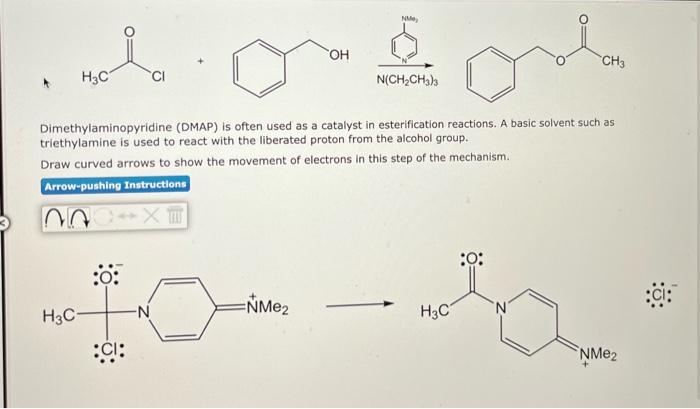
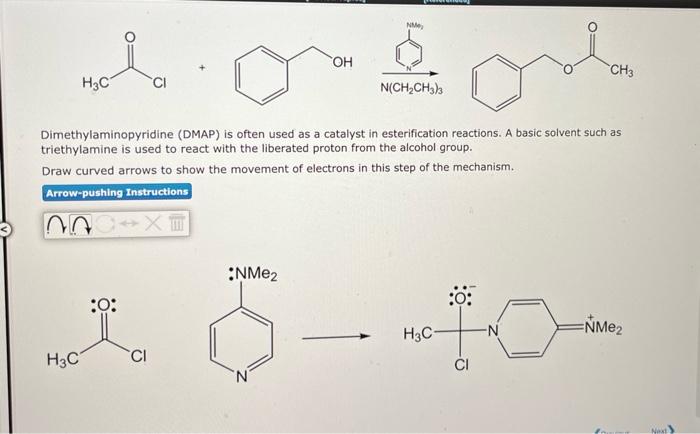
Dimethylaminopyridine (DMAP) is often used as a catalyst in esterification reactions. A basic solvent such as triethylamine is used to react with the liberated proton from the alcohol group. Draw curved arrows to show the movement of electrons in this step of the mechanism.
Dimethylaminopyridine (DMAP) is often used as a catalyst in esterification reactions. A basic solvent such as triethylamine is used to react with the liberated proton from the alcohol group. Draw curved arrows to show the movement of electrons in this step of the mechanism.
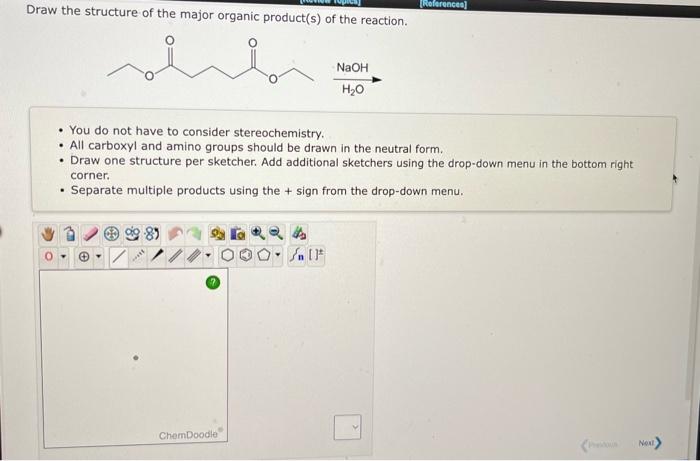
Draw the structure of the major organic product(s) of the reaction. - You do not have to consider stereochemistry. - All carboxyl and amino groups should be drawn in the neutral form. - Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. - Separate multiple products using the \( + \) sign from the drop-down menu.