Home /
Expert Answers /
Chemistry /
please-answer-all-question-below-i-would-really-appreciate-it-they-are-all-connected-nbsp-ka-6-3-pa529
(Solved): please answer all question below i would really appreciate it. they are all connected. ka= 6.3 ...
please answer all question below i would really appreciate it. they are all connected.
![A student prepared a 1:1 buffer, for which [HAc \( ]=\left[\mathrm{Ac}^{-}\right]=0.05290 \mathrm{M} \). Using their \( 1: 1](https://media.cheggcdn.com/study/76b/76b56095-1046-46e9-b10f-fec16228b5e1/image)
![A student prepared a \( 1: 1 \) buffer, for which \( [\mathrm{HAc}]=\left[\mathrm{Ac}^{-}\right]=0.05093 \mathrm{M} \). Using](https://media.cheggcdn.com/study/2c2/2c23efcf-17d1-416a-bedc-e901d48e96fe/image)



![A student prepared a 1:1 buffer, for which [HAc \( ]=\left[\mathrm{Ac}^{-}\right]=0.05290 \mathrm{M} \). Using their \( 1: 1](https://media.cheggcdn.com/study/76b/76b56095-1046-46e9-b10f-fec16228b5e1/image)
![A student prepared a \( 1: 1 \) buffer, for which \( [\mathrm{HAc}]=\left[\mathrm{Ac}^{-}\right]=0.05093 \mathrm{M} \). Using](https://media.cheggcdn.com/study/2c2/2c23efcf-17d1-416a-bedc-e901d48e96fe/image)

ka= 6.3*10^-7
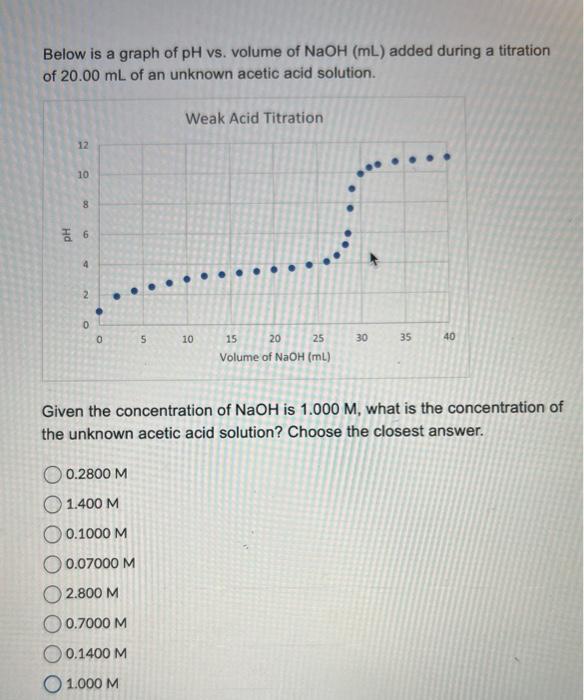
Below is a graph of \( \mathrm{pH} \) vs. volume of \( \mathrm{NaOH}(\mathrm{mL}) \) added during a titration of \( 20.00 \mathrm{~mL} \) of an unknown acetic acid solution. Given the concentration of \( \mathrm{NaOH} \) is \( 1.000 \mathrm{M} \), what is the concentration of the unknown acetic acid solution? Choose the closest answer. \( 0.2800 \mathrm{M} \) \( 1.400 \mathrm{M} \) \( 0.1000 \mathrm{M} \) \( 0.07000 \mathrm{M} \) \( 2.800 \mathrm{M} \) \( 0.7000 \mathrm{M} \) \( 0.1400 \mathrm{M} \) \( 1.000 \mathrm{M} \)
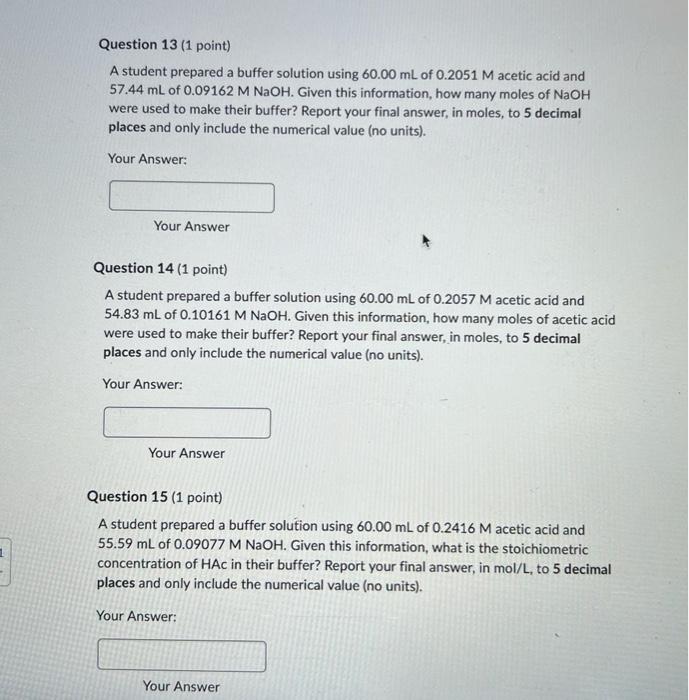
Question 13 (1 point) A student prepared a buffer solution using \( 60.00 \mathrm{~mL} \) of \( 0.2051 \mathrm{M} \) acetic acid and \( 57.44 \mathrm{~mL} \) of \( 0.09162 \mathrm{M} \mathrm{NaOH} \). Given this information, how many moles of \( \mathrm{NaOH} \) were used to make their buffer? Report your final answer, in moles, to 5 decimal places and only include the numerical value (no units). Your Answer: Your Answer Question 14 (1 point) A student prepared a buffer solution using \( 60.00 \mathrm{~mL} \) of \( 0.2057 \mathrm{M} \) acetic acid and \( 54.83 \mathrm{~mL} \) of \( 0.10161 \mathrm{M} \mathrm{NaOH} \). Given this information, how many moles of acetic acid were used to make their buffer? Report your final answer, in moles, to 5 decimal places and only include the numerical value (no units). Your Answer: Your Answer Question 15 (1 point) A student prepared a buffer solution using \( 60.00 \mathrm{~mL} \) of \( 0.2416 \mathrm{M} \) acetic acid and \( 55.59 \mathrm{~mL} \) of \( 0.09077 \mathrm{M} \mathrm{NaOH} \). Given this information, what is the stoichiometric concentration of HAc in their buffer? Report your final answer, in mol/L, to 5 decimal places and only include the numerical value (no units). Your Answer: Your Answer
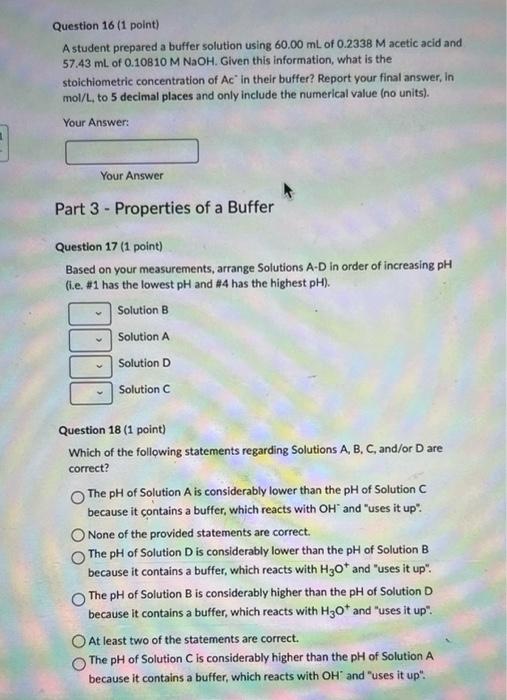
Question 16 (1 point) A student prepared a buffer solution using \( 60.00 \mathrm{~mL} \) of \( 0.2338 \mathrm{M} \) acetic acid and \( 57.43 \mathrm{~mL} \) of \( 0.10810 \mathrm{M} \mathrm{NaOH} \). Given this information, what is the stoichiometric concentration of \( \mathrm{Ac}^{\circ} \) in their buffer? Report your final answer, in mol/ \( / \) L. to 5 decimal places and only include the numerical value (no units). Your Answer: Your Answer Part 3 - Properties of a Buffer Question 17 (1 point) Based on your measurements, arrange Solutions A-D in order of increasing \( \mathrm{pH} \) (i.e. \#1 has the lowest \( \mathrm{pH} \) and \( \# 4 \) has the highest \( \mathrm{pH} \) ). Solution B Solution A Solution D Solution C Question 18 (1 point) Which of the following statements regarding Solutions A, B, C, and/or D are correct? The \( \mathrm{pH} \) of Solution \( \mathrm{A} \) is considerably lower than the \( \mathrm{pH} \) of Solution \( \mathrm{C} \) because it contains a buffer, which reacts with \( \mathrm{OH}^{\text {" }} \) and "uses it up". None of the provided statements are correct. The \( \mathrm{pH} \) of Solution D is considerably lower than the \( \mathrm{pH} \) of Solution B because it contains a buffer, which reacts with \( \mathrm{H}_{3} \mathrm{O}^{+} \)and "uses it up". The \( \mathrm{pH} \) of Solution \( \mathrm{B} \) is considerably higher than the \( \mathrm{pH} \) of Solution \( \mathrm{D} \) because it contains a buffer, which reacts with \( \mathrm{H}_{3} \mathrm{O}^{+} \)and "uses it up". At least two of the statements are correct. The \( \mathrm{pH} \) of Solution \( \mathrm{C} \) is considerably higher than the \( \mathrm{pH} \) of Solution \( \mathrm{A} \) because it contains a buffer, which reacts with \( \mathrm{OH}^{\prime} \) and "uses it up".
A student prepared a 1:1 buffer, for which [HAc \( ]=\left[\mathrm{Ac}^{-}\right]=0.05290 \mathrm{M} \). Using their \( 1: 1 \) buffer and a \( 0.10405 \mathrm{M} \mathrm{NaOH} \) solution, the student prepared Solution D in Part 3 of the experimental procedure. Given this information, how many moles of Ac were there in the student's Solution D before a reaction took place? Report your final answer, in moles, to 6 decimal places and only include the numerical value (no units). Your Answer: Your Answer Question 27 (1 point) A student prepared a 1:1 buffer, for which \( [\mathrm{HAc}]=\left[\mathrm{Ac}^{-}\right]=0.05112 \mathrm{M} \). Using their \( 1: 1 \) buffer and a \( 0.10288 \mathrm{M} \mathrm{HCl} \) solution, the student prepared Solution C in Part 3 of the experimental procedure. Given this information, how many moles of HAc were there in the student's Solution \( \mathrm{C} \) after a reaction took place? Report your final answer, in moles, to 6 decimal places and only include the numerical value (no units). Your Answer: Your Answer
A student prepared a \( 1: 1 \) buffer, for which \( [\mathrm{HAc}]=\left[\mathrm{Ac}^{-}\right]=0.05093 \mathrm{M} \). Using their \( 1: 1 \) buffer and a \( 0.09435 \mathrm{M} \mathrm{HCl} \) solution, the student prepared Solution C in Part 3 of the experimental procedure. Given this information, how many moles of \( \mathrm{Ac}^{-} \)were there in the student's Solution C after a reaction took place? Report your final answer, in moles, to 6 decimal places and only include the numerical value (no units). Your Answer: Your Answer Question 29 (1 point) If the stoichiometric concentrations of \( \mathrm{Ac}^{-} \)and \( \mathrm{HAc} \) in Solution D were known to be \( 0.07278 \mathrm{M} \) and \( 0.02299 \mathrm{M} \), respectively after mixing, then calculate the theoretical \( \mathrm{pH} \) of Solution D after mixing. Use the literature value for the \( \mathrm{pK}_{\mathrm{a}} \) of acetic acid, which is 4.77. Report your final answer to 2 decimal places. Your Answer: Your Answer
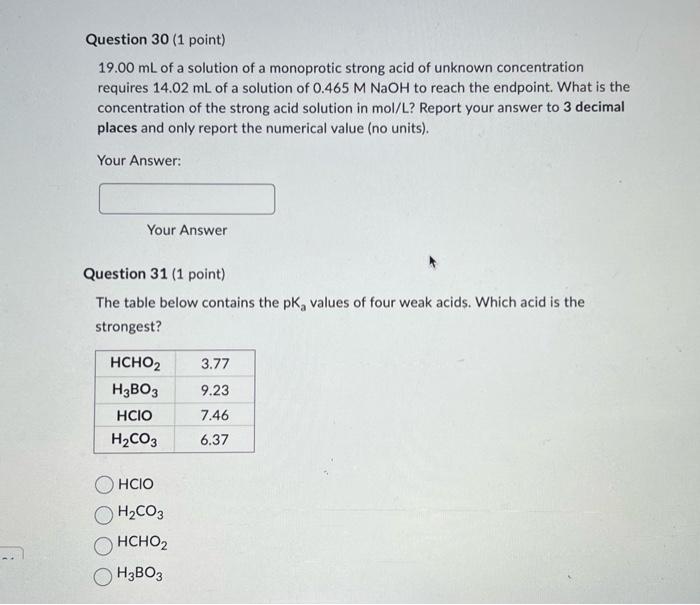
\( 19.00 \mathrm{~mL} \) of a solution of a monoprotic strong acid of unknown concentration requires \( 14.02 \mathrm{~mL} \) of a solution of \( 0.465 \mathrm{M} \mathrm{NaOH} \) to reach the endpoint. What is the concentration of the strong acid solution in mol/L? Report your answer to 3 decimal places and only report the numerical value (no units). Your Answer: Your Answer Question 31 (1 point) The table below contains the \( \mathrm{pK}_{\mathrm{a}} \) values of four weak acids. Which acid is the strongest? \( \mathrm{HClO} \) \( \mathrm{H}_{2} \mathrm{CO}_{3} \) \( \mathrm{HCHO}_{2} \)