Home /
Expert Answers /
Chemistry /
please-also-let-me-know-which-side-the-reaction-would-lie-whether-on-the-reactants-side-or-the-pro-pa550
(Solved): Please also let me know which side the reaction would lie, whether on the reactants side or the pro ...
Please also let me know which side the reaction would lie, whether on the reactants side or the product side, Thank you
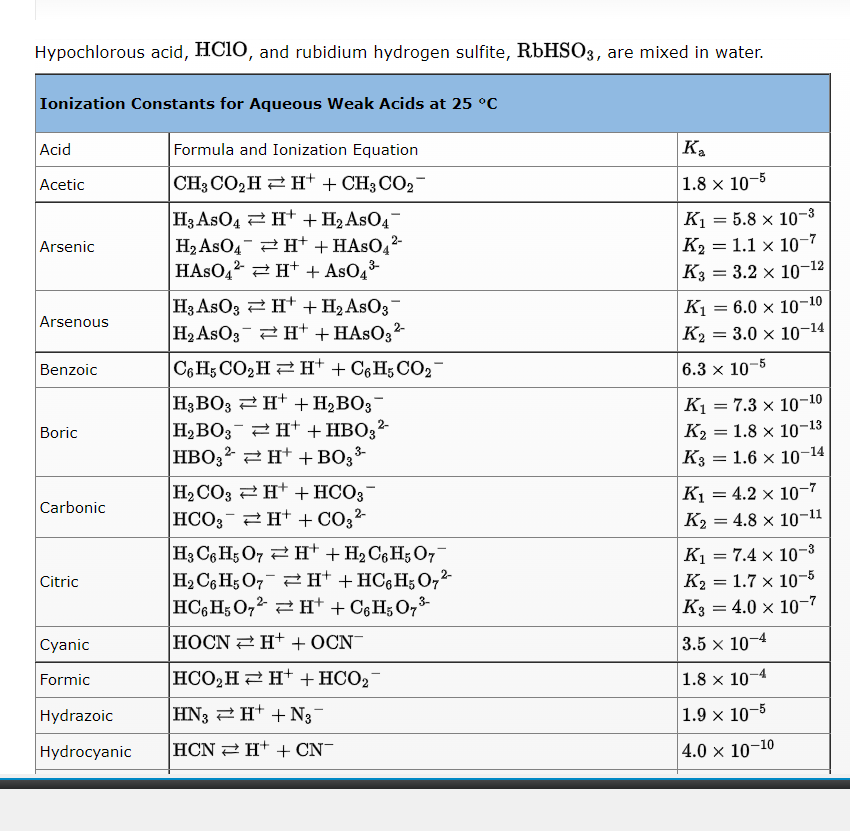
Hypochlorous acid, \( \mathrm{HClO} \), and rubidium hydrogen sulfite, \( \mathrm{RbHSO}_{3} \), are mixed in water.
Choose the balanced, net ionic equation for the acid-base reaction that could, in principle, occur. \[ \begin{array}{l} \mathrm{HClO}(\mathrm{aq})+\mathrm{HSO}_{3}^{-}(\mathrm{aq}) \rightleftarrows \mathrm{ClO}^{-}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{SO}_{3}(\mathrm{aq}) \\ \mathrm{HClO}(\mathrm{aq})+\mathrm{RbHSO}_{3}(\mathrm{aq}) \rightleftarrows \mathrm{RbClO}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{SO}_{3}(\mathrm{aq}) \\ \mathrm{ClO}^{-}(\mathrm{aq})+\mathrm{RbHSO}_{3}(\mathrm{aq}) \rightleftarrows \mathrm{RbClO}(\mathrm{aq})+\mathrm{HSO}_{3}-(\mathrm{aq}) \end{array} \]
Expert Answer
Ans :- Acids are those substances which have tendency to give H+ ions. Strong acid give H+ ion easily because of complete ionization in water and therefore has higher value of acid ioni