Home /
Expert Answers /
Chemistry /
place-the-following-ionic-substances-in-order-from-smallest-to-largest-lattice-energy-cao-csl-kf-pa828
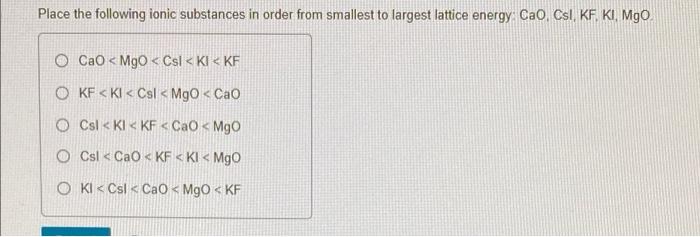
(Solved): Place the following ionic substances in order from smallest to largest lattice energy: CaO, Csl, KF ...
Expert Answer
The correct option is (c). Explanation:- The two primary factors that affect the lattice energy of an ionic compound are :- 1) The magnitude of charge associated with the const