Home /
Expert Answers /
Chemistry /
part-a-write-general-outer-electron-configuration-ns-np-34-for-group-16-in-the-periodic-table-expre-pa373
(Solved): Part A Write general outer-electron configuration (ns np") for group 16 in the periodic table. Expre ...
Part A Write general outer-electron configuration (ns np") for group 16 in the periodic table. Express your answer in order of increasing orbital energy. For example, ns²np² should be entered as ns^2np^2. Submit Request Answer Part B Write general outer-electron configuration (ns* np) for group 17 in the periodic table. Express your answer in order of increasing orbital energy. For example, ns²np² should be entered as ns^2np^2. Submit Part C Request Answer The electron affinity of each group 17 element is greater than that of each corresponding group 16 element Use the electron configurations to explain why this is so. Drag the terms on the left to the appropriate blanks on the right to complete the sentences. three electrons two electrons only one electron no electrons Reset Help 1. The electron affinity of the group 17 elements are more positive than the group 16 elements in the same period because group 17 requires to achieve the noble gas configuration, while the group 16 elements require.
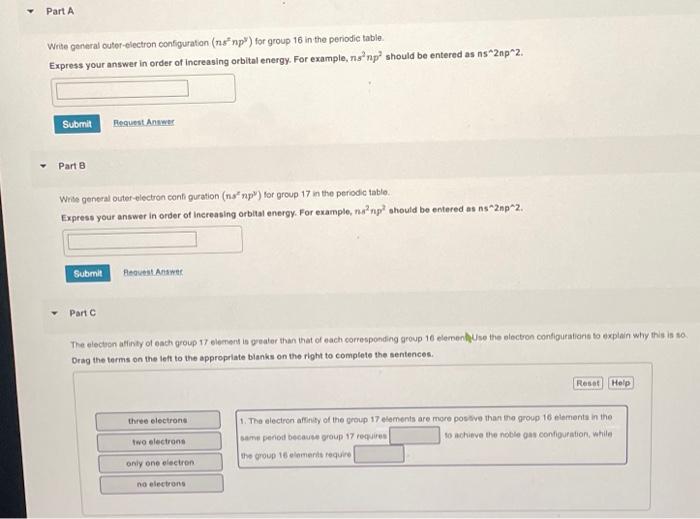
Write general outef-electron configuration for group 16 in the periodic table. Express your answer in order of increasing orbitat energy. For example, should be entered as . Part Wrise general outer-electron cenfi guration ( for group 17 in the periodic table. Express your answer in order of Increasing orbital energy. For example, should be entered as . Part C The electon afinty of each group 17 element is geater thin that of each corresponding group 16 elemenfluse the electron contigurations to explain why this is so Orag the terms on the left to the appropriate bianks on the right to complete the sentences. 1. The electron affinty of the group 17 elements are more postive than the group 16 elements in the same penod because group 17 requires so achieve the noble gas configuration, while The goup 18 elements requine