Home /
Expert Answers /
Chemistry /
part-a-solvent-fp-water-ho-benzene-c6h6-ethanol-ch5oh-carbon-tetrachloride-ccl4-chloro-pa485
(Solved): Part A Solvent fp = Water, HO Benzene, C6H6 Ethanol, CH5OH Carbon tetrachloride, CCL4 Chloro ...
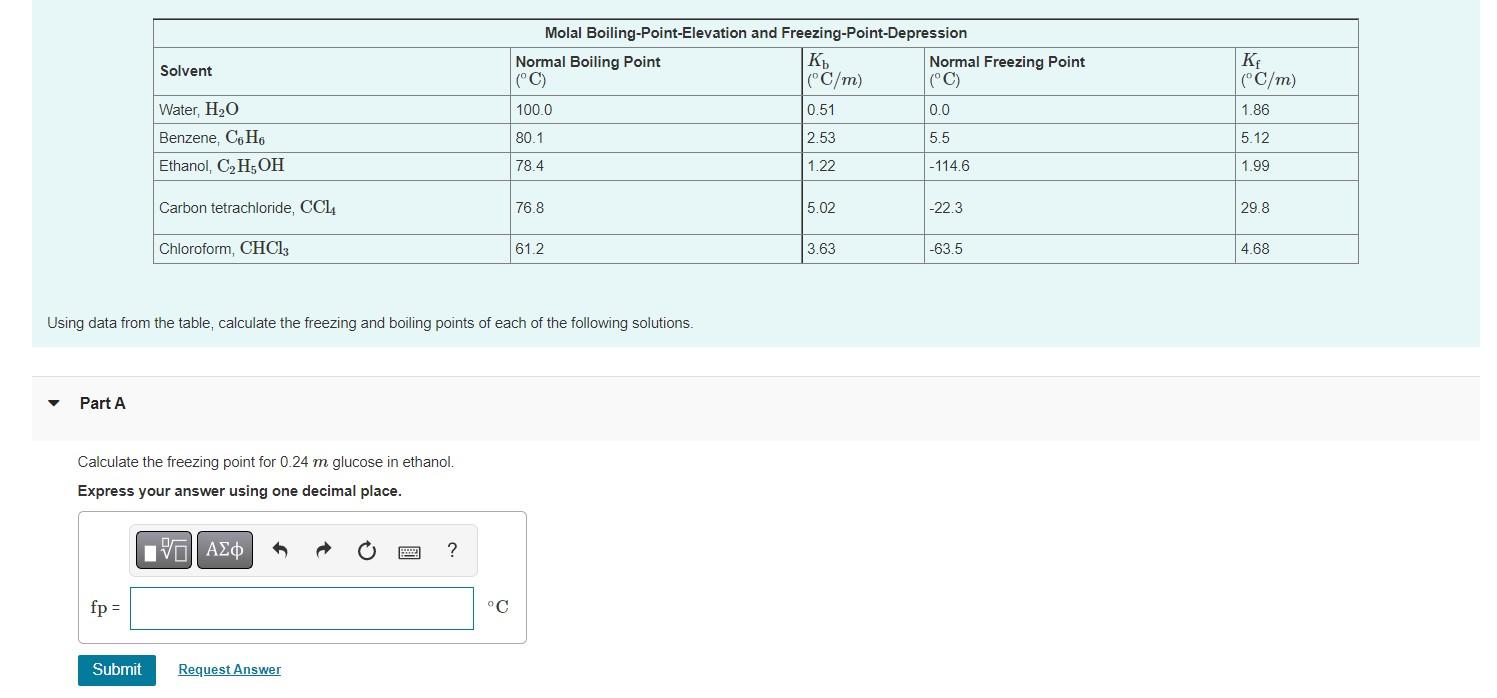
Part A Solvent fp = Water, H?O Benzene, C6H6 Ethanol, C?H5OH Carbon tetrachloride, CCL4 Chloroform, CHC13 Calculate the freezing point for 0.24 m glucose in ethanol. Express your answer using one decimal place. VE ??? Submit Using data from the table, calculate the freezing and boiling points of each of the following solutions. Request Answer ? Molal Boiling-Point-Elevation and Freezing-Point-Depression Normal Boiling Point (°C) 100.0 80.1 78.4 °C 76.8 61.2 Kb (°C/m) 0.51 2.53 1.22 5.02 3.63 Normal Freezing Point (°C) 0.0 5.5 -114.6 -22.3 -63.5 Kf (°C/m) 1.86 5.12 1.99 29.8 4.68
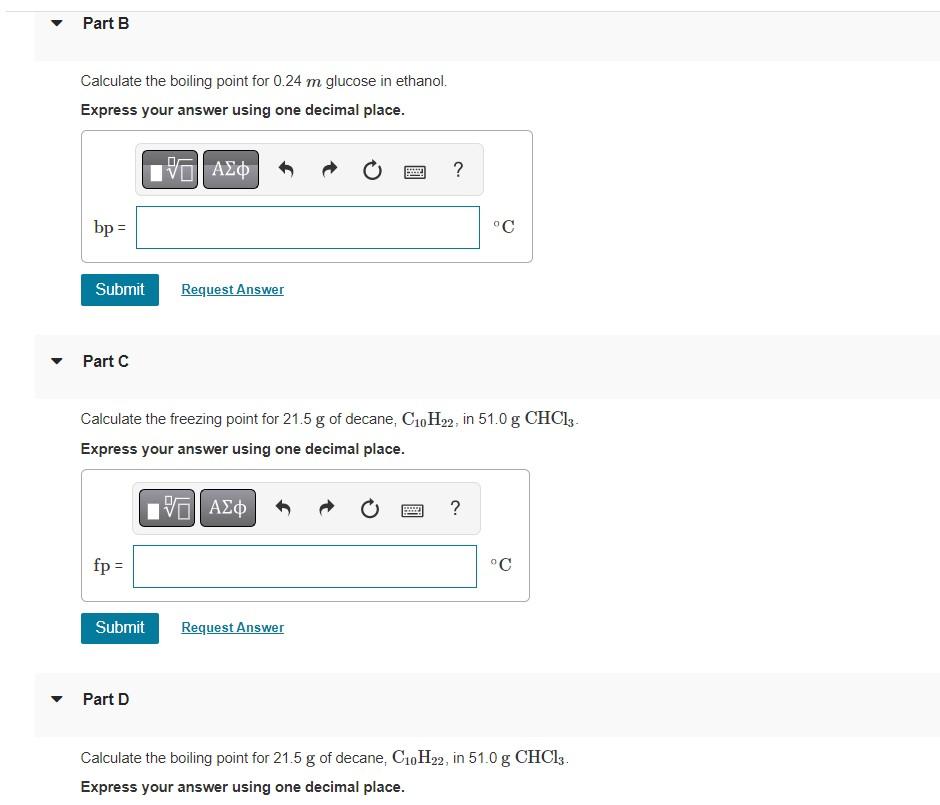
? ? ? Part B Calculate the boiling point for 0.24 m glucose in ethanol. Express your answer using one decimal place. bp= Submit Part C fp = Submit VE ??? | Part D Request Answer Calculate the freezing point for 21.5 g of decane, C?0H?2, in 51.0 g CHC13- Express your answer using one decimal place. G| ??? Request Answer ? d °C ? °C Calculate the boiling point for 21.5 g of decane, C10H22, in 51.0 g CHCl3. Express your answer using one decimal place.
Expert Answer
Part - A ; The parameters are given ; Molality of glucose in ethanol solution = 0.24 m; Freezing point of ethanol = -114.6 oC The Freezing point of a solution containing a non-volatile solute can be expressed as follows: Freezing point of solution =