Home /
Expert Answers /
Chemistry /
part-3-avogadro-39-s-number-estimation-although-avogadro-39-s-number-is-a-huge-number-it-is-possible-pa173
(Solved): Part 3: Avogadro's number estimation Although Avogadro's number is a huge number, it is possible to ...
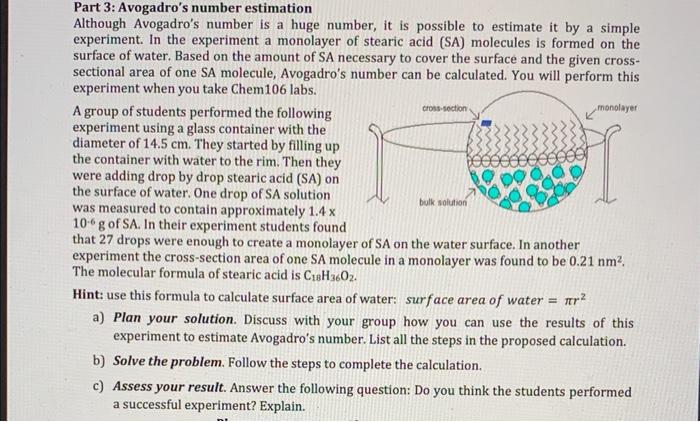
Part 3: Avogadro's number estimation Although Avogadro's number is a huge number, it is possible to estimate it by a simple experiment. In the experiment a monolayer of stearic acid (SA) molecules is formed on the surface of water. Based on the amount of SA necessary to cover the surface and the given crosssectional area of one SA molecule, Avogadro's number can be calculated. You will perform this experiment when you take Chem 106 labs. A group of students performed the following experiment using a glass container with the diameter of \( 14.5 \mathrm{~cm} \). They started by filling up the container with water to the rim. Then they were adding drop by drop stearic acid (SA) on the surface of water. One drop of SA solution was measured to contain approximately \( 1.4 \mathrm{x} \) \( 10^{-6} \mathrm{~g} \) of SA. In their experiment students found that 27 drops were enough to create a monolayer of SA on the water surface. In another experiment the cross-section area of one SA molecule in a monolayer was found to be \( 0.21 \mathrm{~nm}^{2} \). The molecular formula of stearic acid is \( \mathrm{C}_{18} \mathrm{H}_{36} \mathrm{O}_{2} \). Hint: use this formula to calculate surface area of water: surface area of water \( =\pi r^{2} \) a) Plan your solution. Discuss with your group how you can use the results of this experiment to estimate Avogadro's number. List all the steps in the proposed calculation. b) Solve the problem. Follow the steps to complete the calculation. c) Assess your result. Answer the following question: Do you think the students performed a successful experiment? Explain.
Expert Answer
a) SOLUTION PLANNING When Stearic acis is added to water, its molecule will be collected at the water surface.It is assumed that they forms a monolayer. Therefore, the layer is only a molecule thick. The cross sectional area of each Stearic acid mole