Home /
Expert Answers /
Chemistry /
oxidation-and-reduction-assessment-nbsp-lidentify-the-oxidizing-agent-oa-and-reducing-agent-ra-pa195
(Solved): oxidation and reduction assessment lidentify the oxidizing agent (OA) and reducing agent (RA) ...
oxidation and reduction assessment
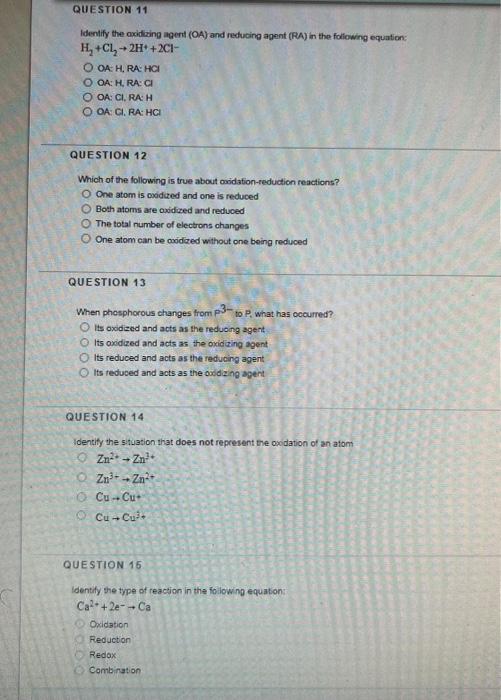

lidentify the oxidizing agent (OA) and reducing agent (RA) in the foliowing equation: \( \mathrm{H}_{2}+\mathrm{Cl}_{2} \rightarrow 2 \mathrm{H}+2 \mathrm{Cl}- \) OA: H, PA: HOI QA: H, RA:CI OA: Cl.RA:H OA: CI. RA: HCI QUESTION 12 Which of the following is true about owidation-reduction reactions? One atom is podued and one is reduced Both atoms are oxidized and reduced The total number of electrons changes One atom can be coidized without one boing reduced QUESTION 13 When phosphorous changes from \( \mathrm{p}^{3} \) - to \( P \). what has ccourred? Its oxdized and acts as the reduong agent. Its oxidized and acts as the oxidaing agent Its reduced and acts as the reducing agent Its redused and acts as the oxd ang agent QUESTION 14 Identify the stuation that does not represent the oxdation of an atom \( 2 n^{2}+2 n^{3}+ \) \( 2 n^{3}+\rightarrow 2 n^{2}+ \) \( \mathrm{Cu}+\mathrm{Cu}+ \) \( \mathrm{Cu} \rightarrow \mathrm{Cu}^{3}+ \) QUESTION 15 Identify the type of reacsion in the following equation: \[ \mathrm{Ca}^{2}+2 e^{-}+\mathrm{Ca} \] Oxidation Peduction Redox Combination
Expert Answer
Answer 11 :- oxidising agent have power of oxidised other atom and reducing agent have power of reducing other atom in this question Cl is oxidising agent and H is reducing agent Option