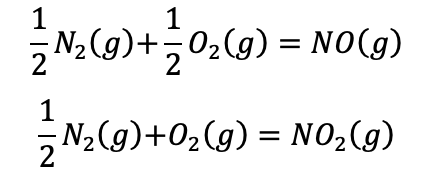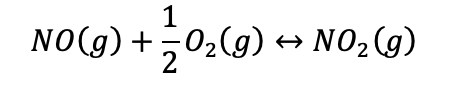(Solved): Nitrogen oxides are a group of gases composed of nitric oxide (NO) and nitrogen dioxide (NO2). The t ...
Nitrogen oxides are a group of gases composed of nitric oxide (NO) and nitrogen dioxide (NO2). The term NOX refers to the combination of both substances. Nitrogen dioxide is the main pollutant of nitrogen oxides, and is formed as a by-product in all combustion carried out at high temperatures.
a) Consider that equilibrium is reached between both nitrogen oxides after combustion in an internal combustion engine at 2,000K and 200 bar, and calculate the mole fractions of NO and NO2 present so that the mole fractions of nitrogen and oxygen in the products of combustion are 0.70 and 0.05.
b) Inhalation of high concentrations of NO2 and for a short
period of time can cause pulmonary edema whose effects are not
observed until a few hours have passed, worsening with physical
exertion. Prolonged exposure can affect the immune system and the
lung, leading to lower resistance to infection and irreversible
changes in lung tissue. NO2 is a precursor for the formation of
photochemical smog, since when combined with other atmospheric
pollutants (VOCs) it influences the reactions of ozone formation on
the earth's surface. NO2 is formed from the oxidation of nitric
oxide (NO), and has a short life in the atmosphere as it is rapidly
oxidized to nitrates (NO3-) or nitric acid. In the latter case, the
phenomenon of acid rain occurs.
The relative compositions of the pollutants NO and NO2 in the air
are governed by the reaction.
Consider that air follows the behavior of an ideal gas and
contains 21 mol% O2 and 79 mol% N2 at 30°C and 1.0133 bar.
i) What is the concentration of NO in parts per million if the
total concentration of the two nitrogen oxides at equilibrium is 5
ppm?
ii) What would be the effect of increasing the pressure on the
compositions at equilibrium? Justify your answer without doing any
calculations.
Expert Answer
a) Let no. of moles of air T0 Initial moles of N2= 0.79To & O2 = 0.21To add both the rea