Home /
Expert Answers /
Chemistry /
nitrogen-gas-can-be-prepared-by-passing-gaseous-ammonia-over-solid-copper-ii-oxide-at-high-temper-pa948
(Solved): Nitrogen gas can be prepared by passing gaseous ammonia over solid copper(II) oxide at high temper ...
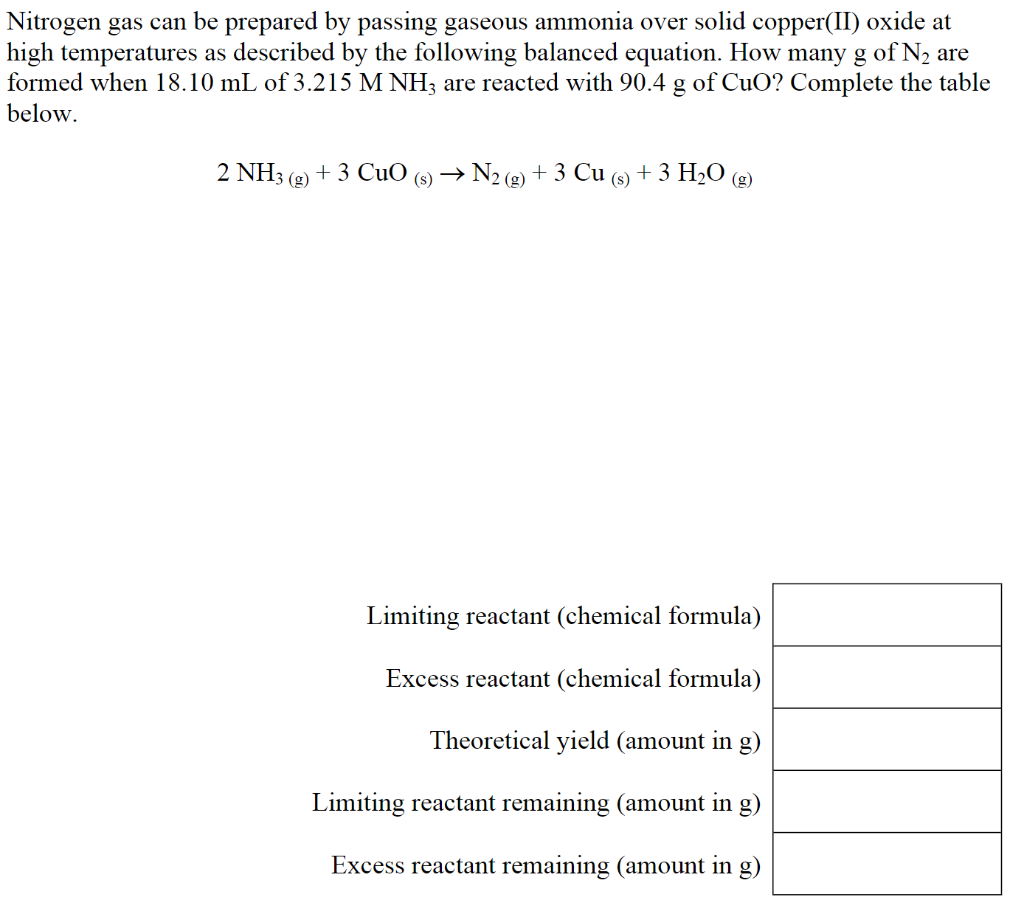
Nitrogen gas can be prepared by passing gaseous ammonia over solid copper(II) oxide at high temperatures as described by the following balanced equation. How many \( g \) of \( \mathrm{N}_{2} \) are formed when \( 18.10 \mathrm{~mL} \) of \( 3.215 \mathrm{M} \mathrm{NH}_{3} \) are reacted with \( 90.4 \mathrm{~g} \) of \( \mathrm{CuO} \) ? Complete the table below. \[ 2 \mathrm{NH}_{3(\mathrm{~g})}+3 \mathrm{CuO}(\mathrm{s}) \rightarrow \mathrm{N}_{2(\mathrm{~g})}+3 \mathrm{Cu}_{(\mathrm{s})}+3 \mathrm{H}_{2} \mathrm{O}_{(\mathrm{g})} \] Limiting reactant (chemical formula) Excess reactant (chemical formula) Theoretical yield (amount in g) Limiting reactant remaining (amount in g) Excess reactant remaining (amount in \( \mathrm{g} \) )