Home /
Expert Answers /
Chemistry /
nitrogen-and-hydrogen-combine-at-a-high-temperature-in-the-presence-of-a-catalyst-to-produce-ammo-pa322
(Solved): Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammo ...

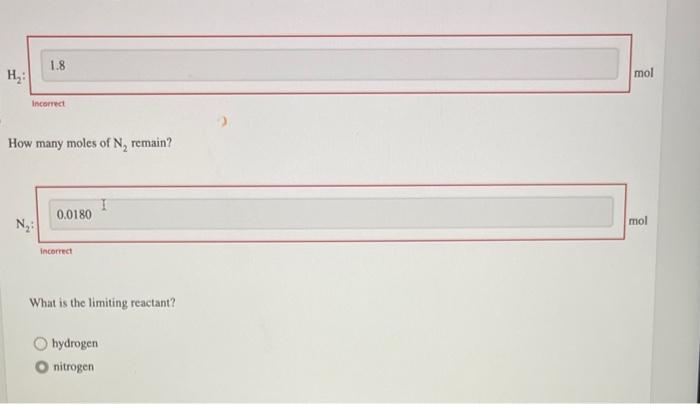
Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia. \[ \mathrm{N}_{2}(\mathrm{~g})+3 \mathrm{H}_{2}(\mathrm{~g}) \longrightarrow 2 \mathrm{NH}_{3}(\mathrm{~g}) \] Assume \( 0.180 \mathrm{~mol} \mathrm{~N}_{2} \) and \( 0.558 \mathrm{~mol} \mathrm{H}_{2} \) are present initially. After complete reaction, how many moles of ammonia are produced? Incorrect How many moles of \( \mathrm{H}_{2} \) remain?
How many moles of \( \mathrm{N}_{2} \) remain? incorrect What is the limiting reactant? hydrogen nitrogen