Home /
Expert Answers /
Advanced Physics /
neglecting-the-spin-of-the-proton-the-angular-momentum-of-hydrogen-is-given-by-the-electron-spin-pa743
(Solved): Neglecting the spin of the proton, the angular momentum of hydrogen is given by the electron spin ...
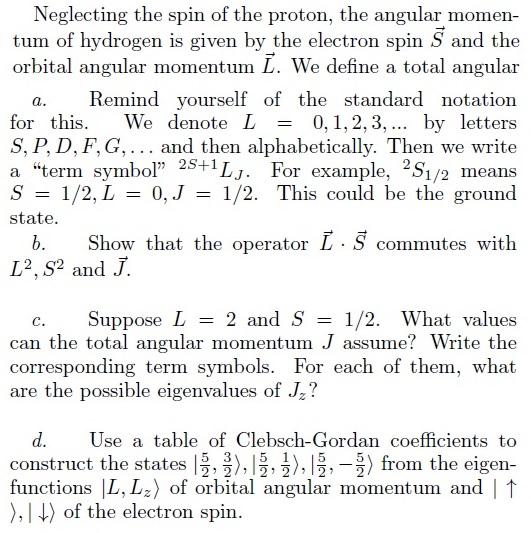
Neglecting the spin of the proton, the angular momentum of hydrogen is given by the electron spin \( \vec{S} \) and the orbital angular momentum \( \vec{L} \). We define a total angular a. Remind yourself of the standard notation for this. We denote \( L=0,1,2,3, \ldots \) by letters \( S, P, D, F, G, \ldots \) and then alphabetically. Then we write a "term symbol" \( 2 S+1 L_{J} \). For example, \( { }^{2} S_{1 / 2} \) means \( S=1 / 2, L=0, J=1 / 2 \). This could be the ground state. b. Show that the operator \( \vec{L} \cdot \vec{S} \) commutes with \( L^{2}, S^{2} \) and \( \vec{J} \) c. Suppose \( L=2 \) and \( S=1 / 2 \). What values can the total angular momentum \( J \) assume? Write the corresponding term symbols. For each of them, what are the possible eigenvalues of \( J_{z} \) ? d. Use a table of Clebsch-Gordan coefficients to construct the states \( \left|\frac{5}{2}, \frac{3}{2}\right\rangle,\left|\frac{5}{2}, \frac{1}{2}\right\rangle,\left|\frac{5}{2},-\frac{5}{2}\right\rangle \) from the eigenfunctions \( \left|L, L_{z}\right\rangle \) of orbital angular momentum and \( \mid \uparrow \) \rangle,\( |\downarrow\rangle \) of the electron spin.