Home /
Expert Answers /
Chemistry /
need-help-q3-compare-the-ir-spectra-of-co-nh3-6-cl3-fig-1-with-co-nh3-5cl3cl2-pa996
(Solved): Need Help! Q3. Compare the IR spectra of [Co(NH3)6]Cl3 (Fig 1) with [Co(NH3)5Cl3Cl2 ...
Need Help!
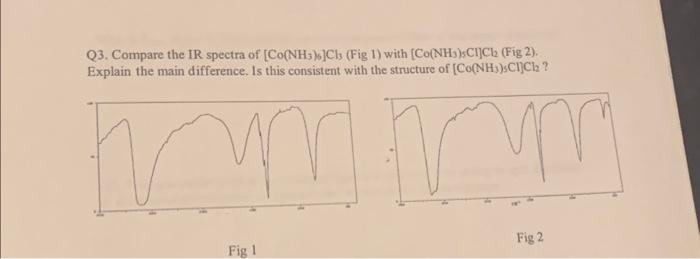


Q3. Compare the IR spectra of (Fig 1) with ( ). Explain the main difference. Is this consistent with the structure of ? Fig 1 Fig 2
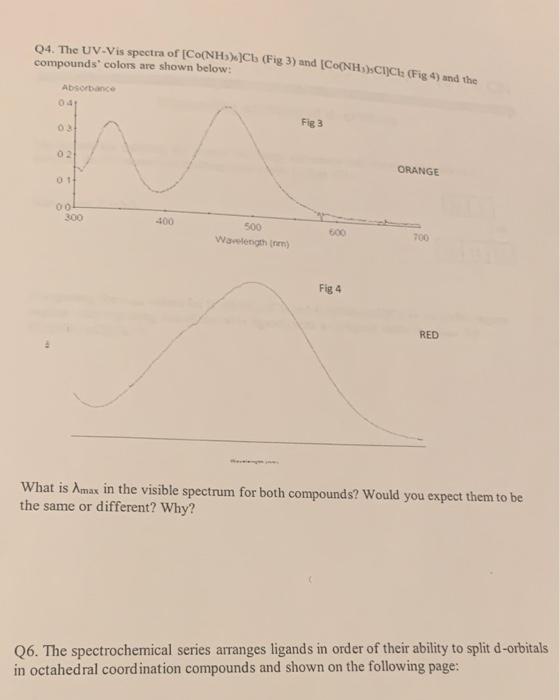
Q4. The UV-Vis spectra of (Fig 3) and (Fie compounds' colors are shown below: What is in the visible spectrum for both compounds? Would you expect them to be the same or different? Why? Q6. The spectrochemical series arranges ligands in order of their ability to split d-orbitals in octahedral coordination compounds and shown on the following page:
Increasing splitting of d-orbitals The greater the splitting, the greater the energy difference between the sets of orbitals. So causes less splitting (and lower ) than . Also recall Comparing the values for and in the visible region, explain why replacing one of the ligands with a ligand shifts the for ?