Home /
Expert Answers /
Chemistry /
nbsp-the-combustion-shown-below-of-ethane-left-mathrm-c-2-mathrm-h-6-right-wi-pa631
(Solved): The combustion (shown below) of ethane \( \left(\mathrm{C}_{2} \mathrm{H}_{6}\right) \) wi ...
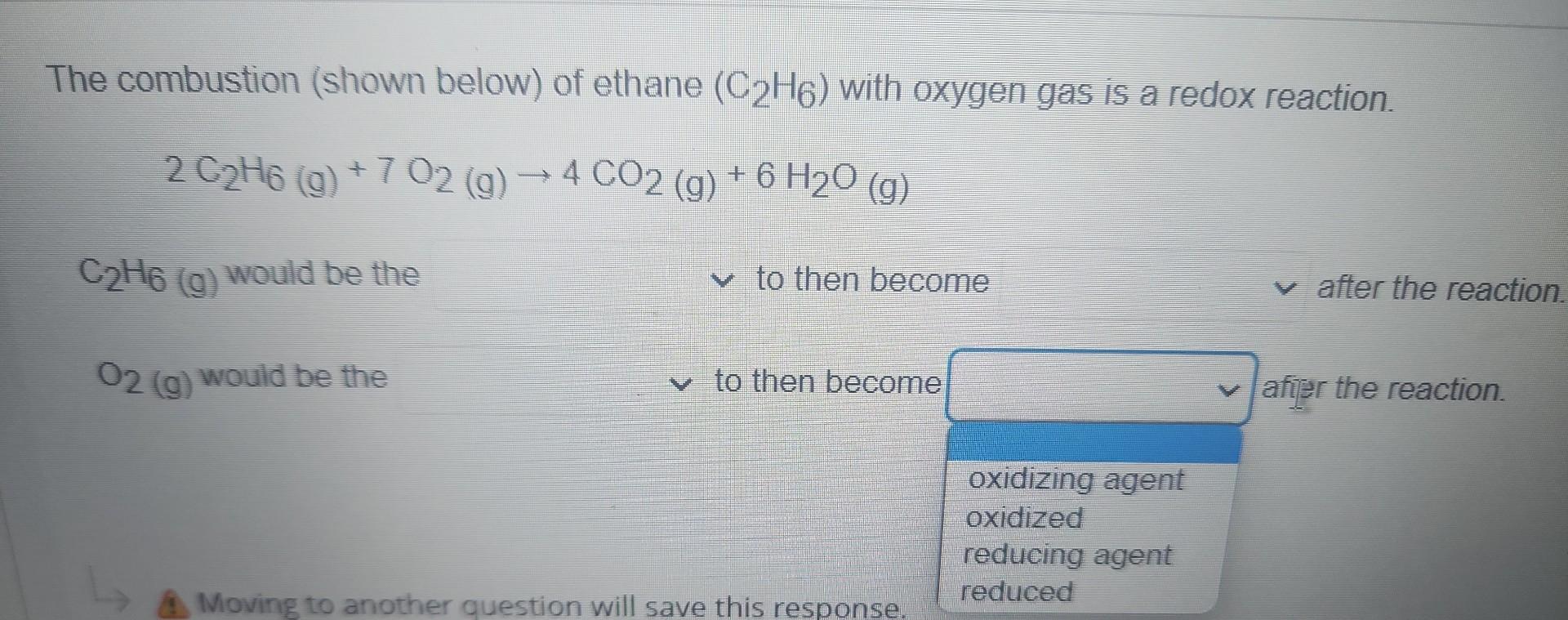
The combustion (shown below) of ethane \( \left(\mathrm{C}_{2} \mathrm{H}_{6}\right) \) with oxygen gas is a redox reaction. \[ 2 \mathrm{C}_{2} \mathrm{H}_{6}(\mathrm{~g})+7 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow 4 \mathrm{CO}_{2}(\mathrm{~g})+6 \mathrm{H}_{2} \mathrm{O}(g) \] \( \mathrm{C}_{2} \mathrm{H}_{6}(\mathrm{~g}) \) would be the to then become after the reaction. \( \mathrm{O}_{2}(\mathrm{~g}) \) would be the to then become afier the reaction.