Home /
Expert Answers /
Chemistry /
nbsp-nbsp-the-image-below-shows-the-free-energy-diagrams-for-four-different-reactions-a-b-pa523
(Solved): The image below shows the free energy diagrams for four different reactions (A, B ...
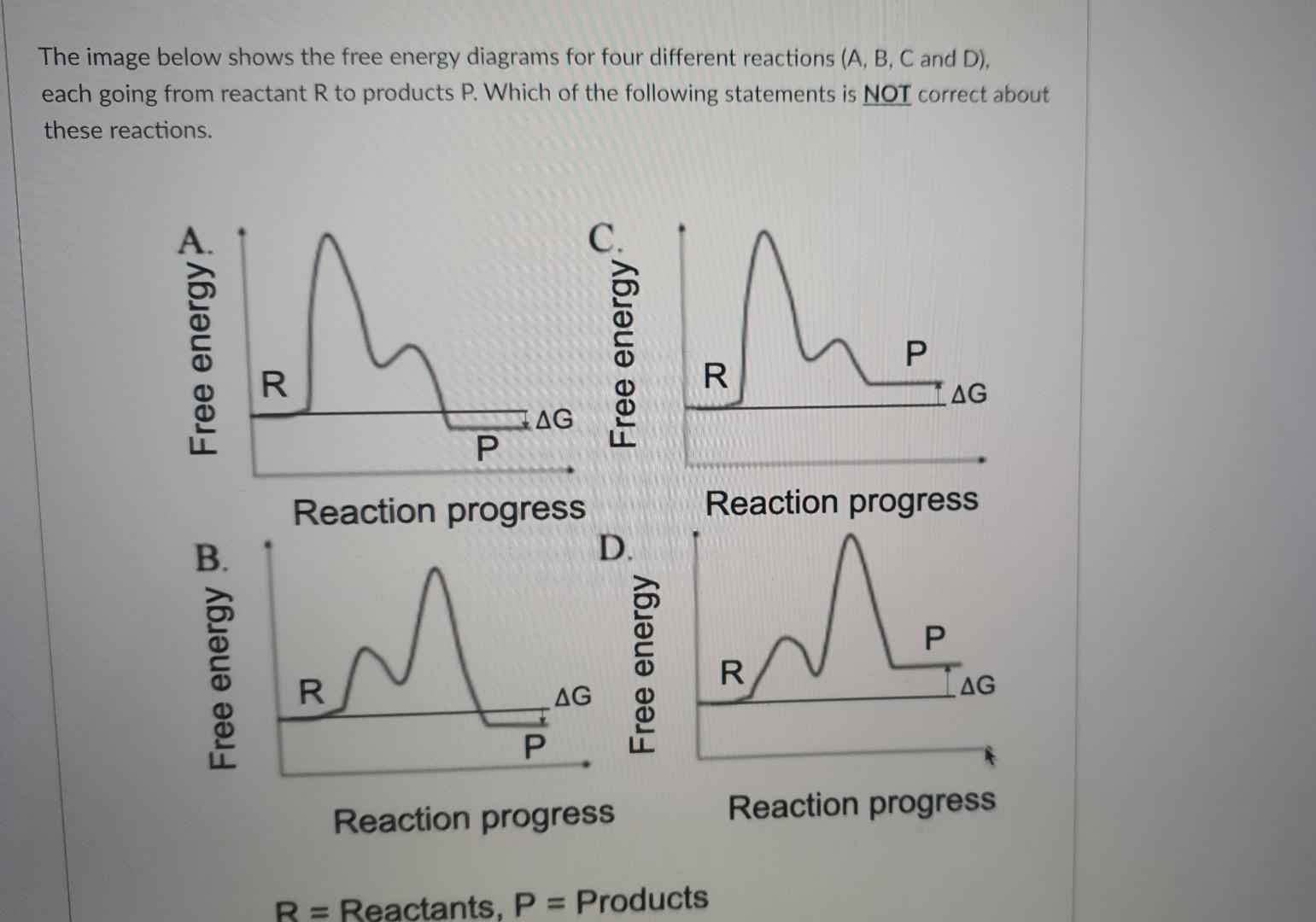

The image below shows the free energy diagrams for four different reactions (A, B, C and D), each going from reactant R to products P. Which of the following statements is NOT correct about these reactions. P Free energy? B. Free energy R AG P Reaction progress R AG Free energy D. R AG Reaction progress P R AG Reaction progress P Reaction progress R = Reactants, P = Products
Reactions C and D are both energetically unfavourable Reactions C and D are both unspontaneous Reactions A and B are both endergonic Reactions A and B are both spontaneous O Reactions A and B are both exergonic
Expert Answer
Solution:- 3 no. Statement is incorrect. Explanation 1 no. Statement (reaction C and D are both energetically unfavourable) :- energytically unfavourable reaction means a chemical reaction in which standard change in free energy is positive and an ad