Home /
Expert Answers /
Chemistry /
nbsp-in-ionic-compounds-quad-lose-their-valence-electrons-to-form-positively-charged-me-pa410
(Solved): In ionic compounds, \( \quad \) lose their valence electrons to form positively charged me ...
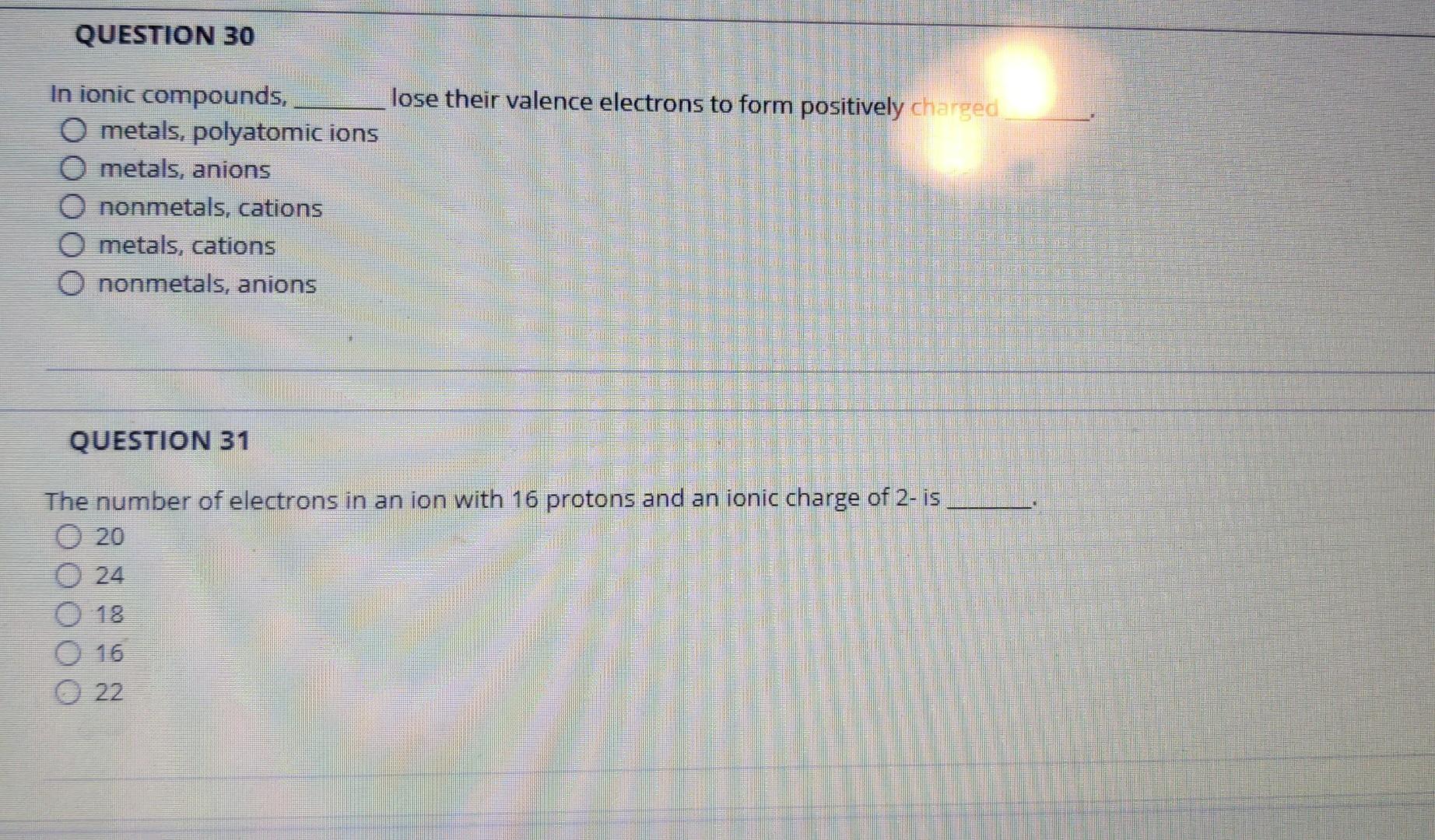
In ionic compounds, \( \quad \) lose their valence electrons to form positively charged metals, polyatomic ions metals, anions nonmetals, cations metals, cations nonmetals, anions QUESTION 31 The number of electrons in an ion with 16 protons and an ionic charge of 2-is 20 24 18 16 22
Expert Answer
Q30) ans- 4th option is correct. Metals & cations. Metals dontes their electrons from outermost shell to complete their octet and forms positively charge