Home /
Expert Answers /
Chemistry /
nbsp-i-need-help-with-the-lewis-structures-hybridization-of-atom-and-molecular-geometry-of-sod-pa737
(Solved): I need help with the lewis structures, hybridization of atom, and molecular geometry of sod ...
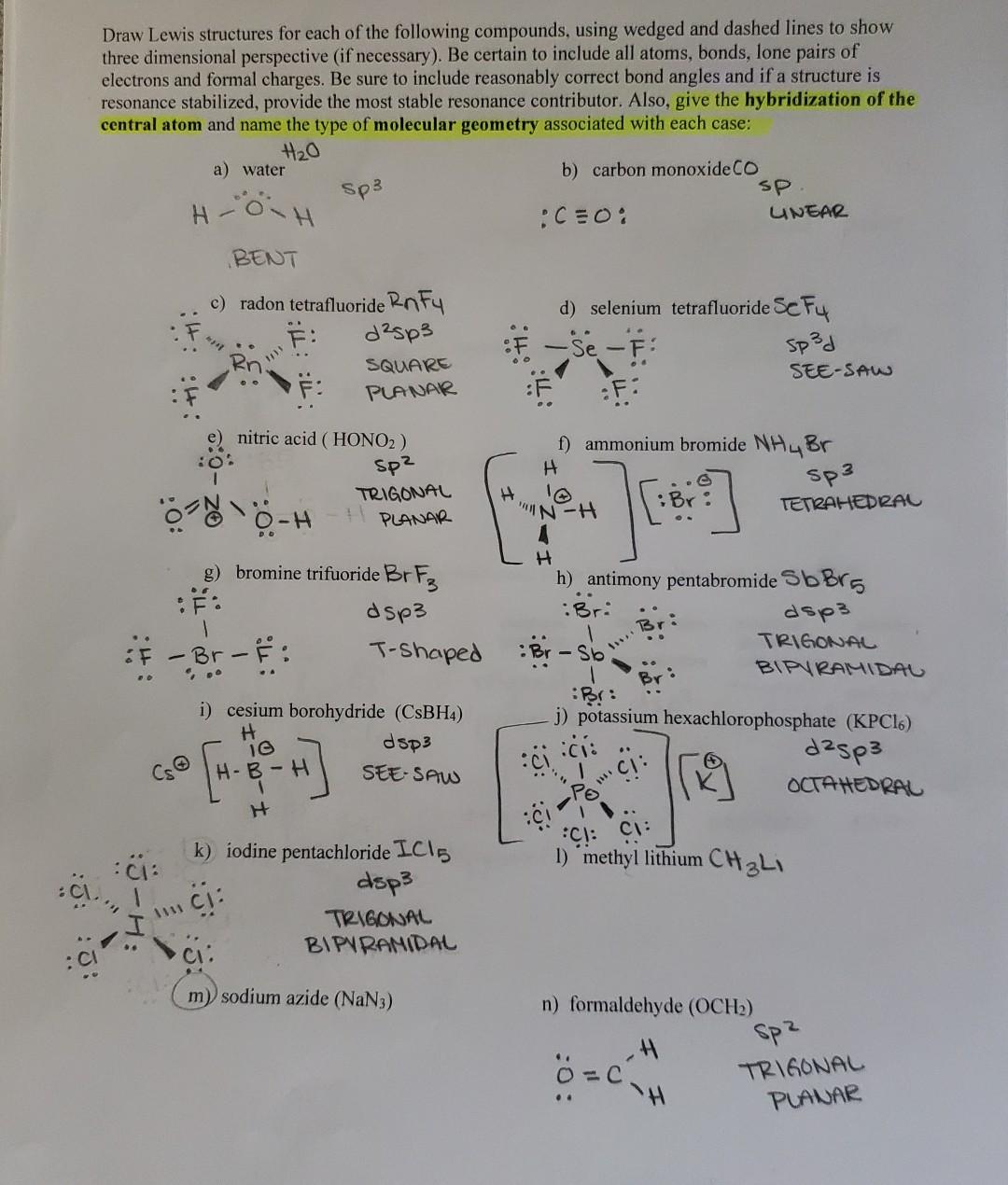
I need help with the lewis structures, hybridization of atom, and molecular geometry of sodium azide (NaN³) and methyl lithium (CH³Li). thanks for the help in advanced
Draw Lewis structures for each of the following compounds, using wedged and dashed lines to show three dimensional perspective (if necessary). Be certain to include all atoms, bonds, lone pairs of electrons and formal charges. Be sure to include reasonably correct bond angles and if a structure is resonance stabilized, provide the most stable resonance contributor. Also, give the hybridization of the central atom and name the type of molecular geometry associated with each case: a) water b) carbon monoxide \( \mathrm{CO} \) \( H \rightarrow \operatorname{OP}^{3} \) \( : C \equiv 0: \) sp BENT c) radon tetrafluoride \( \mathrm{R}_{\cap} \mathrm{F}_{4} \) d) selenium tetrafluoride Se \( F_{4} \) e) nitric acid \( \left(\mathrm{HONO}_{2}\right) \) \( \left.\begin{array}{ccc}\because \% & \text { SP } & \text { f) ammonium bromide } \mathrm{NH}_{4} \mathrm{Br} \\ 1 & \text { TRIGONAL }\end{array}\right] \begin{array}{c}\mathrm{H} \\ \ldots\end{array} \) planar TETRAHEDRAL i) cesium borohydride \( \left(\mathrm{CsBH}_{4}\right) \) j) potassium hexachlorophosphate \( \left(\mathrm{KPCl}_{6}\right) \) OCTAHEDRAL : 1 Cl: \( \ddot{\mathrm{C}}: \) dsp 3 1) methyl lithium \( \mathrm{CH}_{3} \mathrm{Ll}_{1} \) TRIGONAL \( \ddot{C I} \) : BIPYRAMIDAL m) sodium azide \( \left(\mathrm{NaN}_{3}\right) \) n) formaldehyde \( \left(\mathrm{OCH}_{2}\right) \)