Home /
Expert Answers /
Chemistry /
nbsp-consider-the-reaction-of-hydrochloric-acid-molar-mass-is-36-5-mathrm-g-and-cal-pa990
(Solved): Consider the reaction of hydrochloric acid (molar mass is \( 36.5 \mathrm{~g} \) ) and cal ...
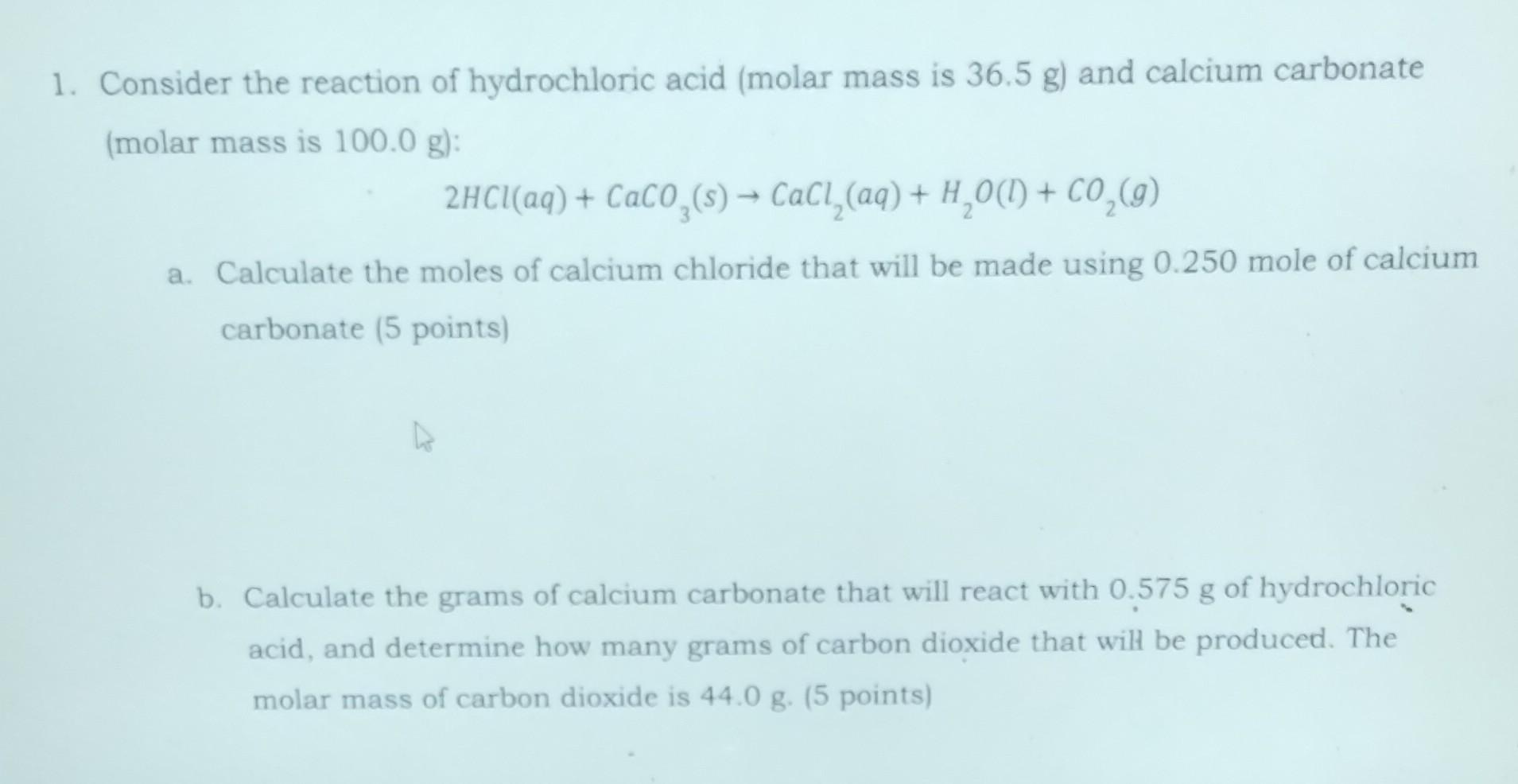
Consider the reaction of hydrochloric acid (molar mass is \( 36.5 \mathrm{~g} \) ) and calcium carbonate (molar mass is \( 100.0 \mathrm{~g} \) ): \[ 2 \mathrm{HCl}(a q)+\mathrm{CaCO}_{3}(s) \rightarrow \mathrm{CaCl}_{2}(a q)+\mathrm{H}_{2} \mathrm{O}(\mathrm{l})+\mathrm{CO}_{2}(g) \] a. Calculate the moles of calcium chloride that will be made using \( 0.250 \) mole of calcium carbonate (5 points) b. Calculate the grams of calcium carbonate that will react with \( 0.575 \mathrm{~g} \) of hydrochloric acid, and determine how many grams of carbon dioxide that will be produced. The molar mass of carbon dioxide is \( 44.0 \mathrm{~g} \). (5 points)
Expert Answer
a. To calculate the moles of calcium chloride that will be made using 0.250 mole