Home /
Expert Answers /
Chemistry /
nbsp-consider-the-insoluble-compound-silver-hydroxide-agoh-the-silver-ion-also-forms-a-comp-pa566
(Solved): Consider the insoluble compound silver hydroxide, AgOH. The silver ion also forms a comp ...
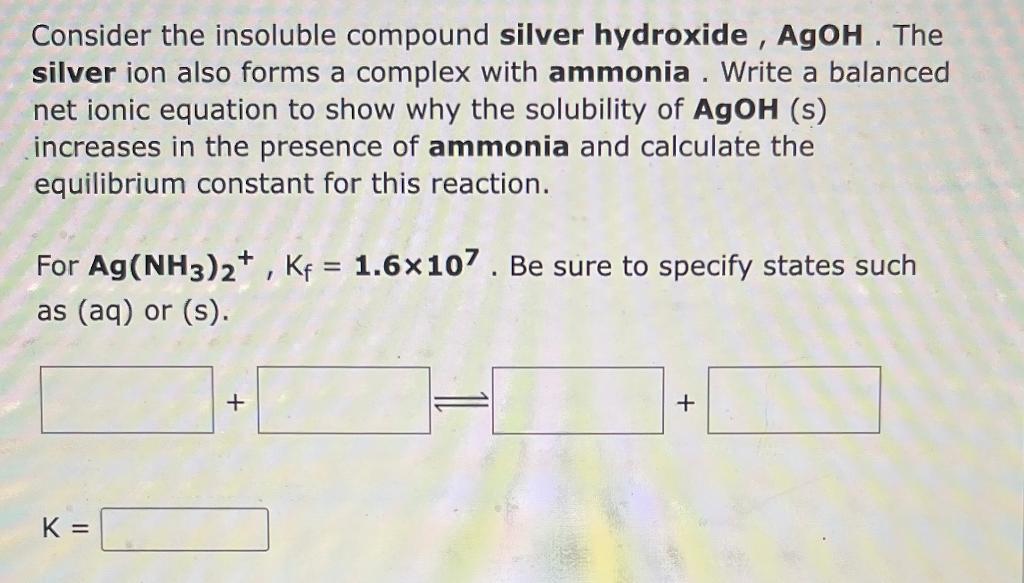
Consider the insoluble compound silver hydroxide, AgOH. The silver ion also forms a complex with ammonia. Write a balanced net ionic equation to show why the solubility of \( \mathbf{A g O H} \) (s) increases in the presence of ammonia and calculate the equilibrium constant for this reaction. For \( \mathbf{A g}\left(\mathbf{N H}_{3}\right)_{2}^{+}, \mathrm{K}_{\mathrm{f}}=\mathbf{1 . 6} \times 10^{7} \). Be sure to specify states such as (aq) or \( (\mathrm{s}) \). \[ \mathrm{K}= \]
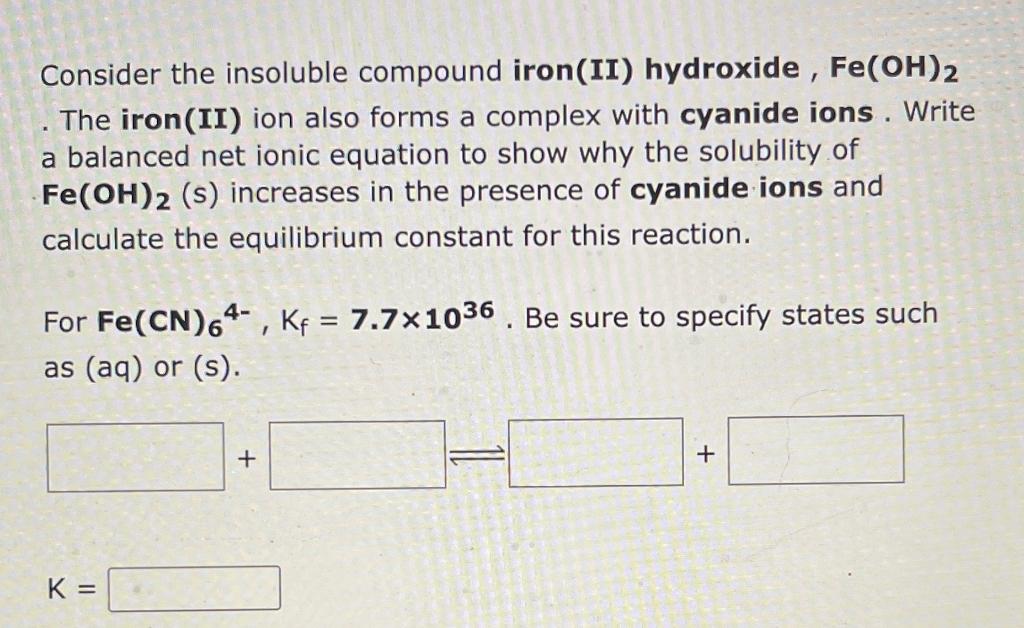
Consider the insoluble compound iron(II) hydroxide, \( \mathrm{Fe}(\mathrm{OH})_{2} \) The iron(II) ion also forms a complex with cyanide ions. Write a balanced net ionic equation to show why the solubility of \( \mathrm{Fe}(\mathrm{OH})_{2} \) (s) increases in the presence of cyanide ions and calculate the equilibrium constant for this reaction. For \( \mathbf{F e}(\mathrm{CN})_{6}^{4^{-}}, \mathrm{K}_{\mathrm{f}}=\mathbf{7 . 7} \times 10^{36} \). Be sure to specify states such as \( (\mathrm{aq}) \) or \( (\mathrm{s}) \). \[ \mathrm{K}= \]
Expert Answer
Solution: 1st question. Balanced chemical equation is; AgOH(s) + 2NH3(aq)