Home /
Expert Answers /
Chemistry /
nbsp-consider-the-balanced-reversible-reaction-of-acetic-acid-with-ethanol-which-takes-place-w-pa256
(Solved): Consider the balanced reversible reaction of acetic acid with ethanol, which takes place w ...
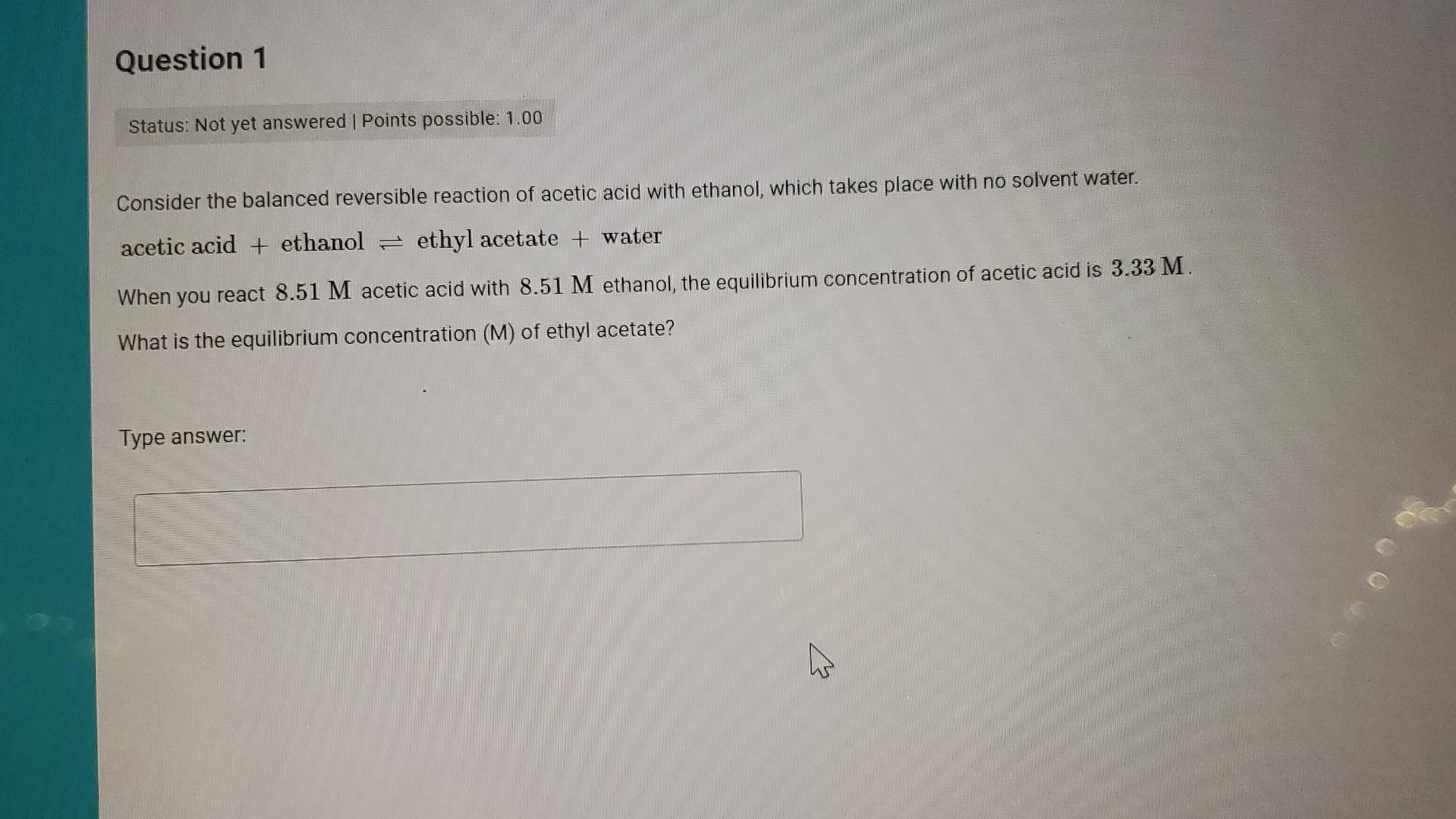
Consider the balanced reversible reaction of acetic acid with ethanol, which takes place with no solvent water. acetic acid + ethanol \( \rightleftharpoons \) ethyl acetate + water When you react \( 8.51 \mathrm{M} \) acetic acid with \( 8.51 \mathrm{M} \) ethanol, the equilibrium concentration of acetic acid is \( 3.33 \mathrm{M} \). What is the equilibrium concentration \( (M) \) of ethyl acetate?