Home /
Expert Answers /
Chemistry /
nbsp-calculate-the-equilibrium-constant-k-for-the-following-reaction-mathrm-ag-le-pa217
(Solved): Calculate the equilibrium constant \( (K) \) for the following reaction: \[ \mathrm{Ag}\le ...
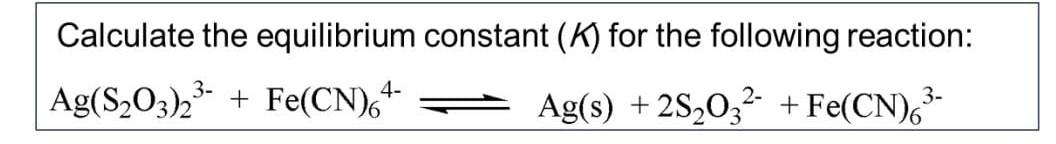
Calculate the equilibrium constant \( (K) \) for the following reaction: \[ \mathrm{Ag}\left(\mathrm{S}_{2} \mathrm{O}_{3}\right)_{2}{ }^{3-}+\mathrm{Fe}(\mathrm{CN})_{6}{ }^{4-} \rightleftharpoons \mathrm{Ag}(\mathrm{s})+2 \mathrm{~S}_{2} \mathrm{O}_{3}{ }^{2-}+\mathrm{Fe}(\mathrm{CN})_{6}{ }^{3-} \]
Expert Answer
Given, To calculate the equilibrium constant (k) for the reaction Ag(S2O3)23- + Fe(CN)64- <------> Ag(s) + 2S2O32- + Fe(CN)63- we take general value