Home /
Expert Answers /
Chemistry /
nbsp-based-on-the-following-reduction-potential-data-which-is-the-strongest-reducing-agent-pa432
(Solved): Based on the following reduction potential data, which is the strongest reducing agent? \[ ...
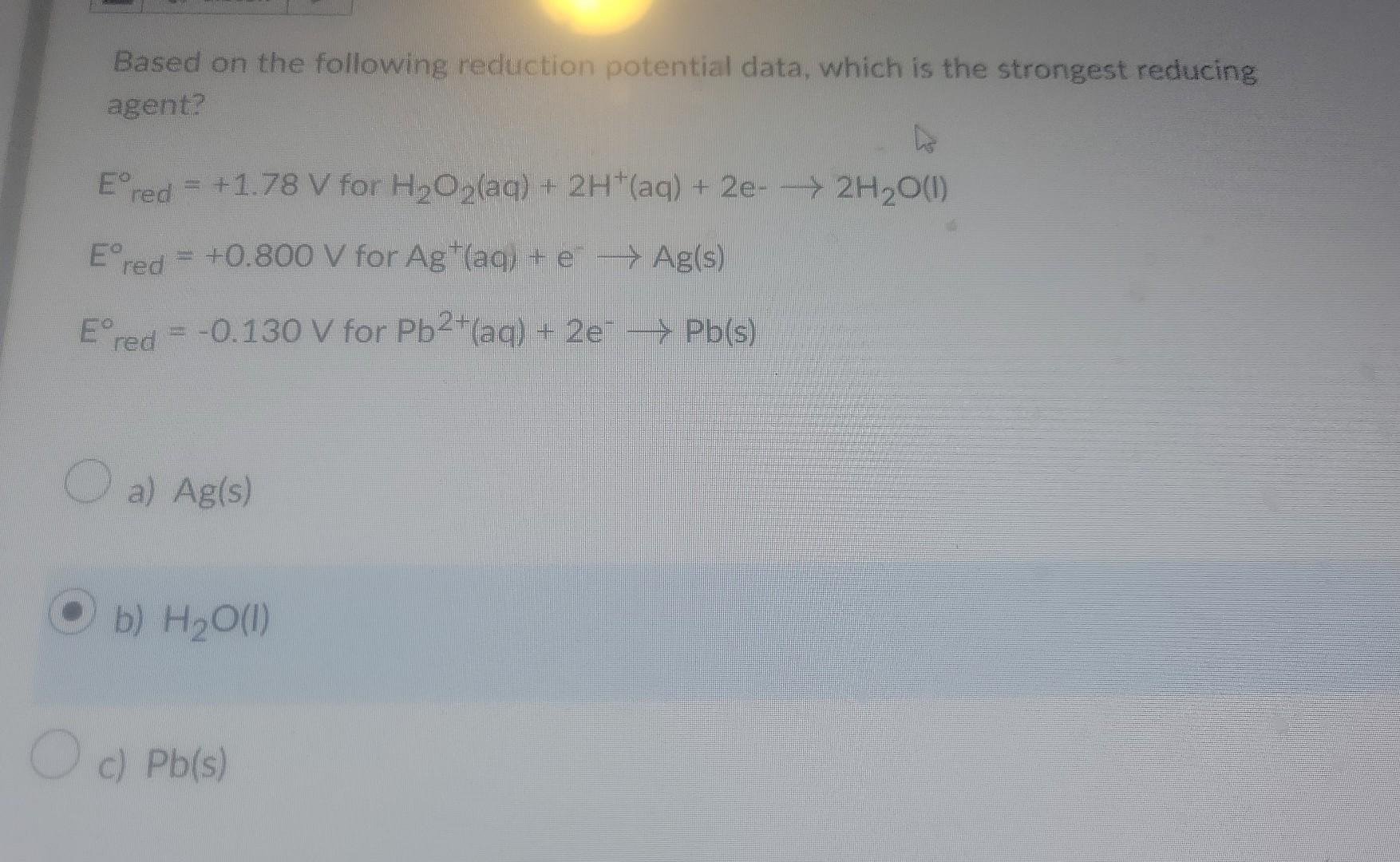
Based on the following reduction potential data, which is the strongest reducing agent? \[ \begin{array}{l} E_{\text {red }}^{\circ}=+1.78 \mathrm{~V} \text { for } \mathrm{H}_{2} \mathrm{O}_{2}(\mathrm{aq})+2 \mathrm{H}^{+}(\mathrm{aq})+2 \mathrm{e}_{-} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \\ E_{\text {red }}^{\circ}=+0.800 \mathrm{~V} \text { for } \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ag}(\mathrm{s}) \\ E_{\text {red }}^{\circ}=-0.130 \mathrm{~V} \text { for } \mathrm{Pb}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Pb}(\mathrm{s}) \end{array} \] a) \( \mathrm{Ag}(\mathrm{s}) \) b) \( \mathrm{H}_{2} \mathrm{O}(\mathrm{I}) \) c) \( \mathrm{Pb}(\mathrm{s}) \)
Expert Answer
Oxidation : Oxidation is defined as the process by which an atom or ion loses one or more electrons or increases in oxidation state. As per the old definition, oxidation is the addition of oxygen or any electronegative element or the removal of hydro