Home /
Expert Answers /
Chemistry /
nbsp-balance-the-following-chemical-equation-then-answer-the-following-question-mathrm-c-pa889
(Solved): Balance the following chemical equation, then answer the following question. \[ \mathrm{C} ...
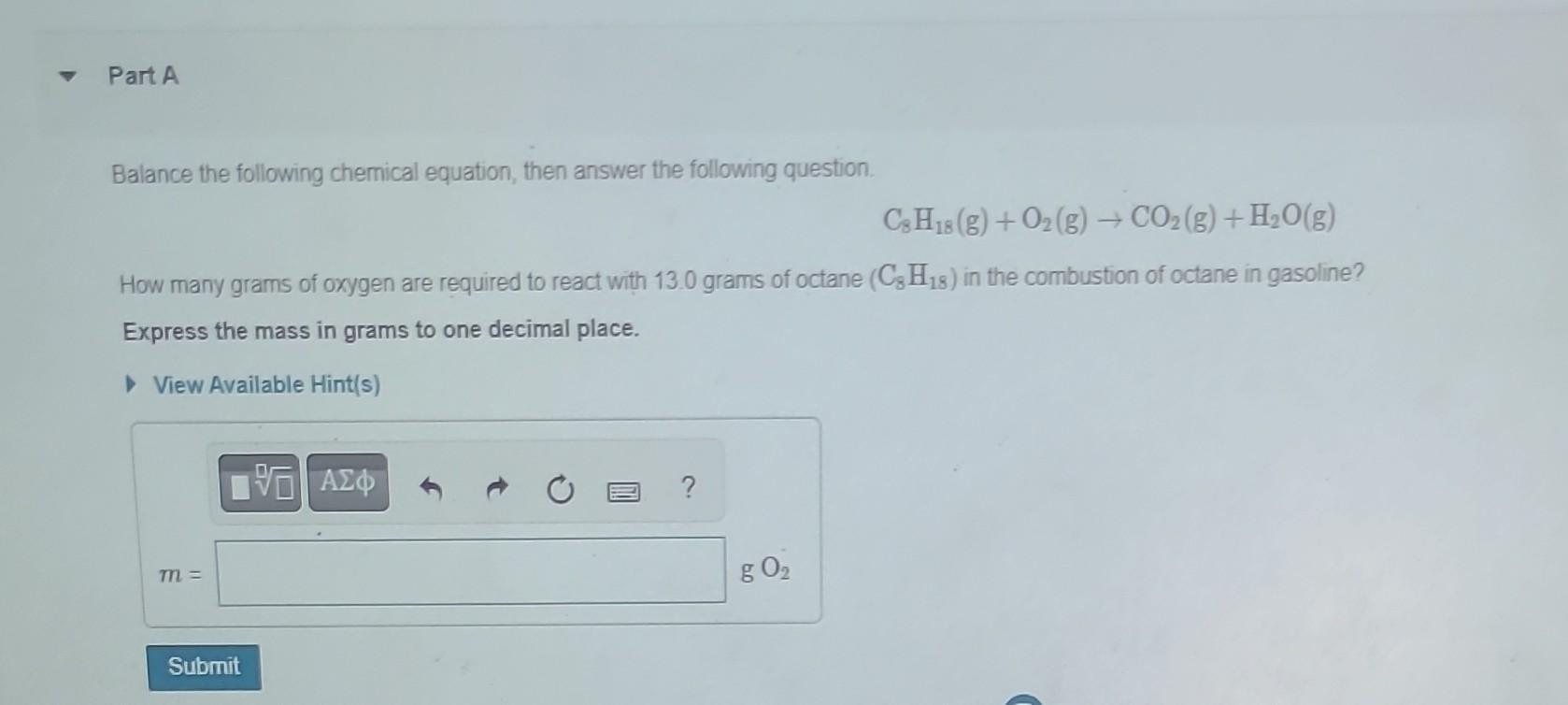
Balance the following chemical equation, then answer the following question. \[ \mathrm{C}_{8} \mathrm{H}_{18}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CO}_{2}(\mathrm{~g})+\mathrm{H}_{2} \mathrm{O}(\mathrm{g}) \] How many grams of oxygen are required to react with \( 13.0 \) grams of octane \( \left(\mathrm{C}_{8} \mathrm{H}_{18}\right) \) in the combustion of octane in gasoline? Express the mass in grams to one decimal place.
Expert Answer
Given that the equation C8H18(g