Home /
Expert Answers /
Chemistry /
nbsp-a-4-272-g-sample-of-a-mixture-containing-only-mathrm-cu-2-mathrm-o-and-mat-pa792
(Solved): A 4.272-g sample of a mixture containing only \( \mathrm{Cu}_{2} \mathrm{O} \) and \( \mat ...
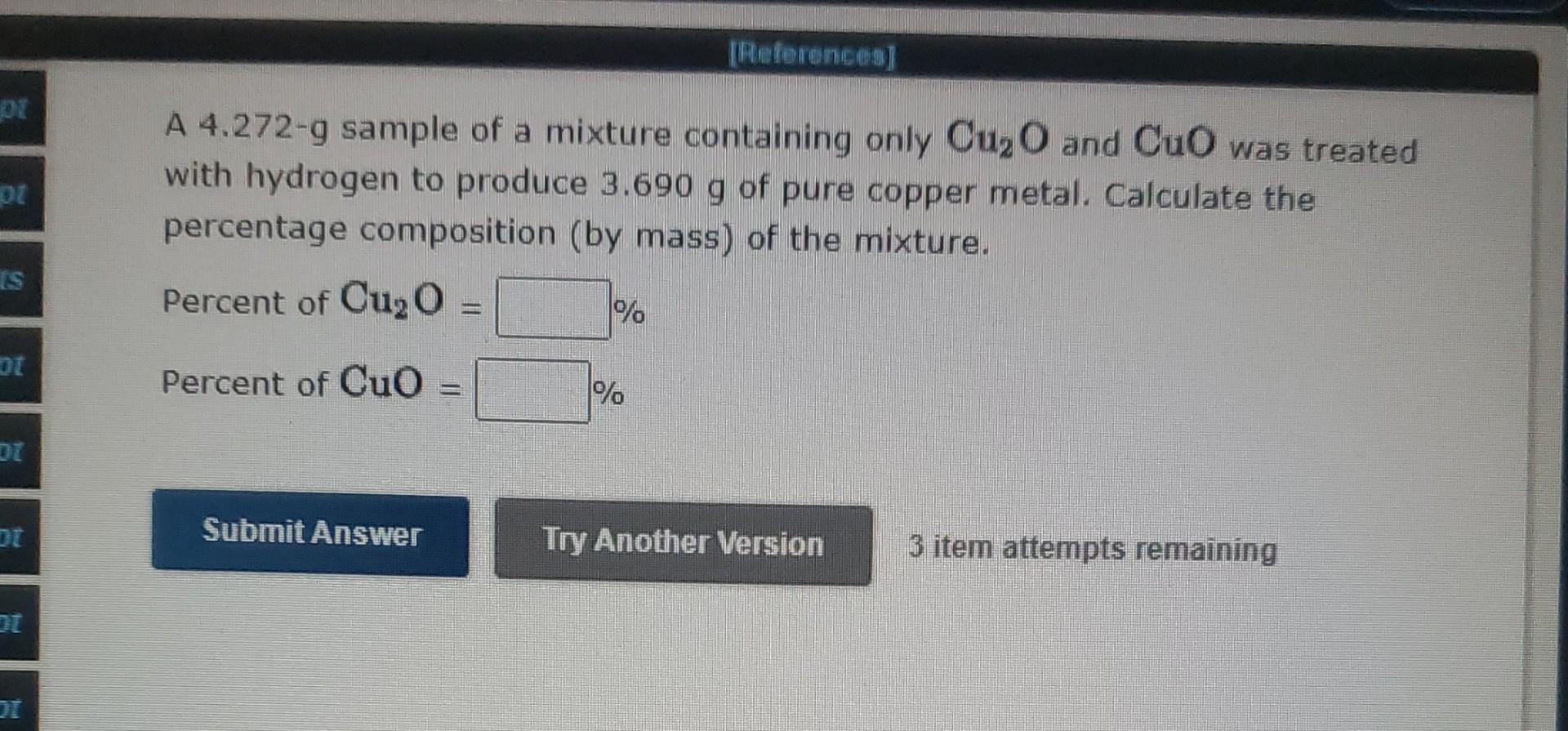
A 4.272-g sample of a mixture containing only \( \mathrm{Cu}_{2} \mathrm{O} \) and \( \mathrm{CuO} \) was treated with hydrogen to produce \( 3.690 \mathrm{~g} \) of pure copper metal. Calculate the percentage composition (by mass) of the mixture. Percent of \( \mathrm{Cu}_{2} \mathrm{O}= \) Percent of \( \mathrm{CuO}= \) 3 item attempts remaining
Expert Answer
Solution: Mass of Cu2O and CuO mixture = 4.272g Mass of Cu = 3.690g The reaction of mixture with hydrogen is, Cu2O + H2