Home /
Expert Answers /
Chemistry /
naturally-occurring-cobalt-consists-of-only-one-isotope-59co-whose-relative-atomic-mass-is-find-pa325
(Solved): Naturally occurring cobalt consists of only one isotope, 59Co, whose relative atomic mass is Find ...
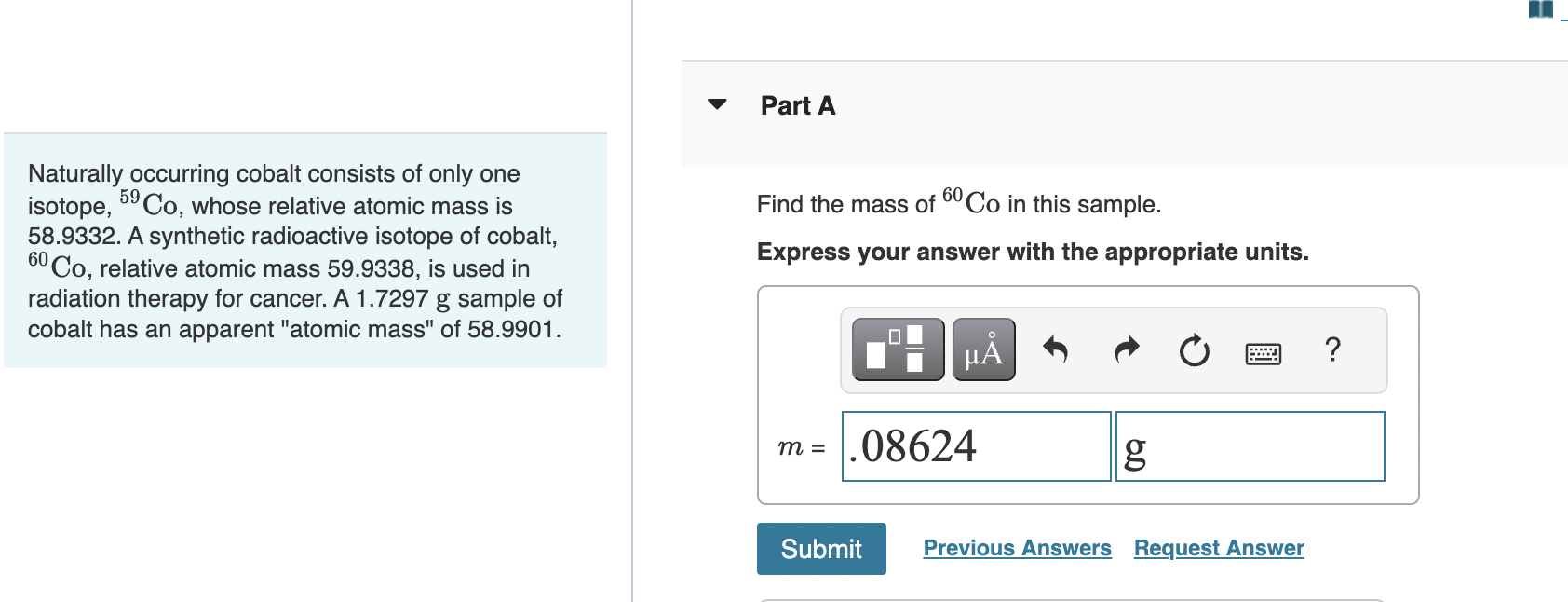
Naturally occurring cobalt consists of only one isotope, , whose relative atomic mass is Find the mass of in this sample. 58.9332. A synthetic radioactive isotope of cobalt, , relative atomic mass , is used in Express your answer with the appropriate units. radiation therapy for cancer. A sample of cobalt has an apparent "atomic mass" of .
Expert Answer
58.9901 = 58.9332 × X + 59.9338 × 100 - X / 10