Home /
Expert Answers /
Chemical Engineering /
naoh-c2h5-ch3co-gt-na-ch3co-c2h5oh-nbsp-the-liquid-phase-reaction-is-carried-out-in-a-b-pa218
(Solved): NaOH + C2H5(CH3CO) -----> Na(CH3CO) + C2H5OH The liquid phase reaction is carried out in a b ...
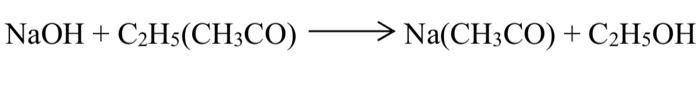
NaOH + C2H5(CH3CO) -----> Na(CH3CO) + C2H5OH
The liquid phase reaction is carried out in a batch reactor. Explain in detail the necessary steps to prepare the sodium hydroxide and ethyl acetate solutions with a concentration of 1.25 M each and a total volume of 1.5 liters of the reaction solution in the reactor and make the NECESSARY calculations.
Molecular mass of ethyl acetate: 88.11 g/mol Molecular mass of sodium hydroxide: 40 g/mol
\( \mathrm{NaOH}+\mathrm{C}_{2} \mathrm{H}_{5}\left(\mathrm{CH}_{3} \mathrm{CO}\right) \longrightarrow \mathrm{Na}\left(\mathrm{CH}_{3} \mathrm{CO}\right)+\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH} \)
Expert Answer
Given, Ethyl acetate formula is C2H5(CH3COO). Please check, Molecular weight is correct. NaOH+C2H5(CH3COO)?Na(CH3COO)+C2H5OH Molecular weight of NaOH=