Home /
Expert Answers /
Chemical Engineering /
n-pentane-is-burned-with-excess-air-in-a-continuous-combustion-chamber-below-is-a-skeleton-flowch-pa269
(Solved): n-Pentane is burned with excess air in a continuous combustion chamber. Below is a skeleton flowch ...
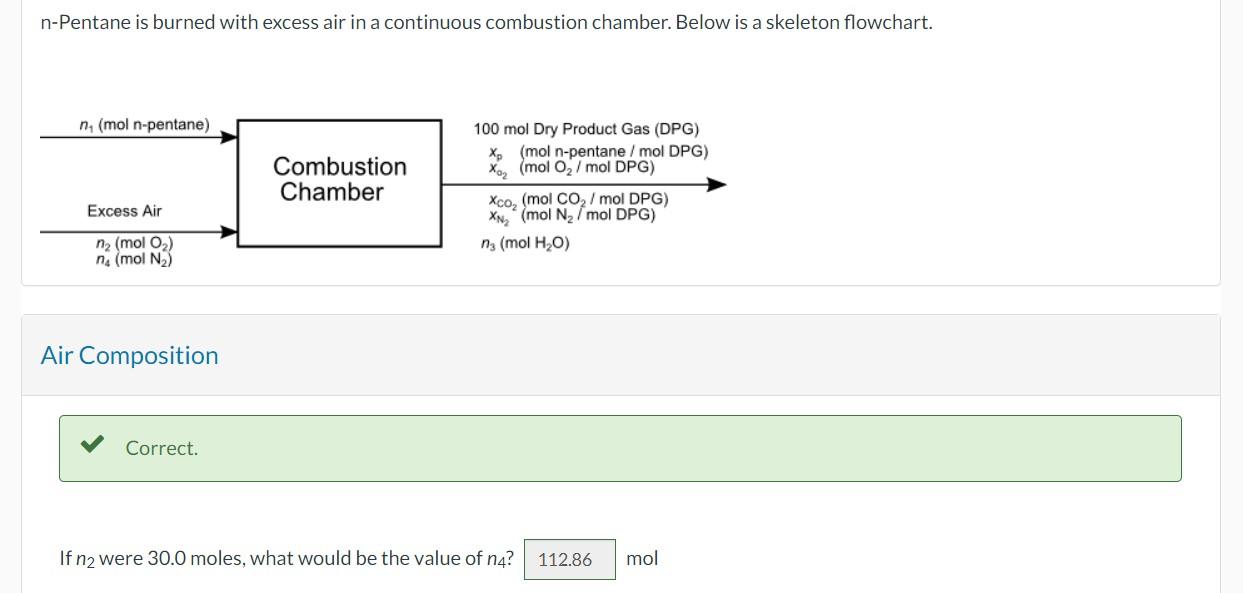
-Pentane is burned with excess air in a continuous combustion chamber. Below is a skeleton flowchart. Air Composition If were 30.0 moles, what would be the value of ?
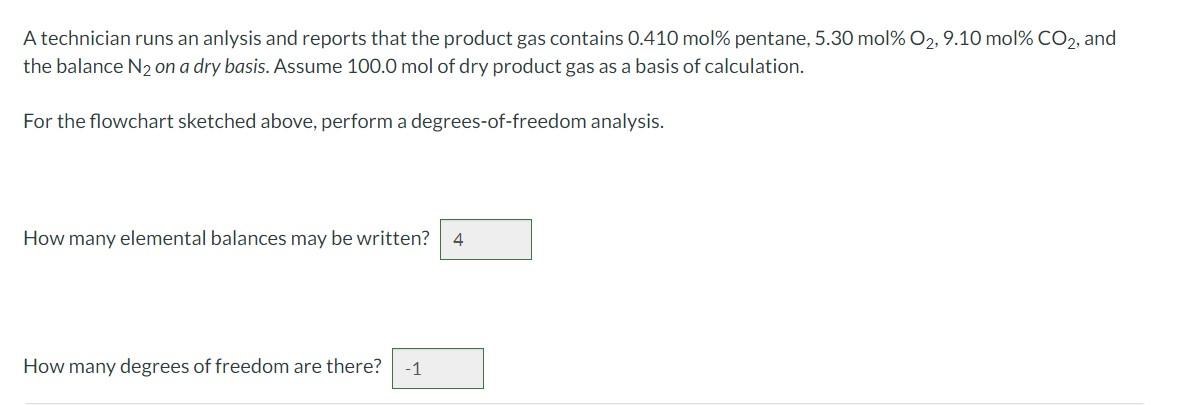
A technician runs an anlysis and reports that the product gas contains pentane, , and the balance on a dry basis. Assume 100.0 mol of dry product gas as a basis of calculation. For the flowchart sketched above, perform a degrees-of-freedom analysis. How many elemental balances may be written? How many degrees of freedom are there?
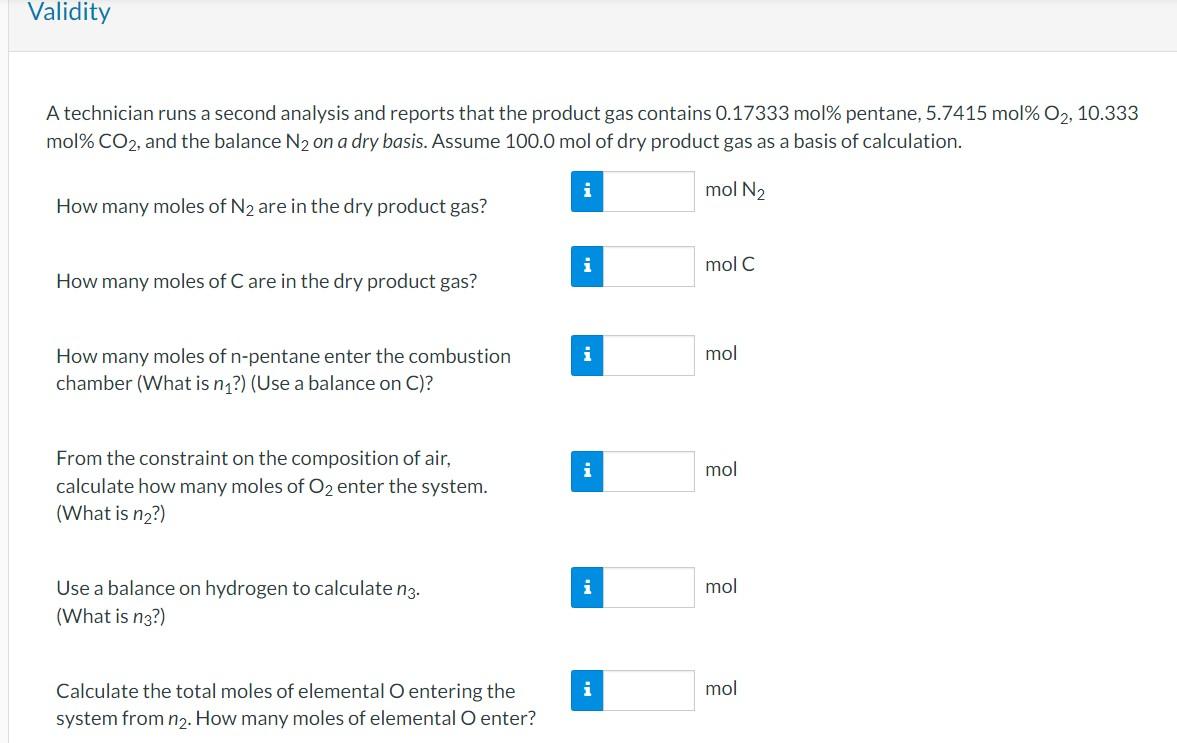
A technician runs an analysis and reports that the product gas contains pentane, , and the balance on a dry basis. Assume 100.0 mol of dry product gas as a basis of calculation. How many moles of are in the dry product gas? How many moles of are in the dry product gas? How many moles of -pentane enter the combustion chamber (What is ?) (Use a balance on C)? From the constraint on the composition of air, calculate how many moles of enter the system. (What is ?) Use a balance on hydrogen to calculate . What is Calculate the total moles of elemental entering the system from . How many moles of elemental enter? Calculate the total moles of elemental O leaving the system
A technician runs a second analysis and reports that the product gas contains pentane, mol\% , and the balance on a dry basis. Assume of dry product gas as a basis of calculation. How many moles of are in the dry product gas? How many moles of are in the dry product gas? How many moles of -pentane enter the combustion chamber (What is ?) (Use a balance on C)? From the constraint on the composition of air, calculate how many moles of enter the system. (What is ?) Use a balance on hydrogen to calculate . (What is ?) Calculate the total moles of elemental entering the system from . How many moles of elemental enter?
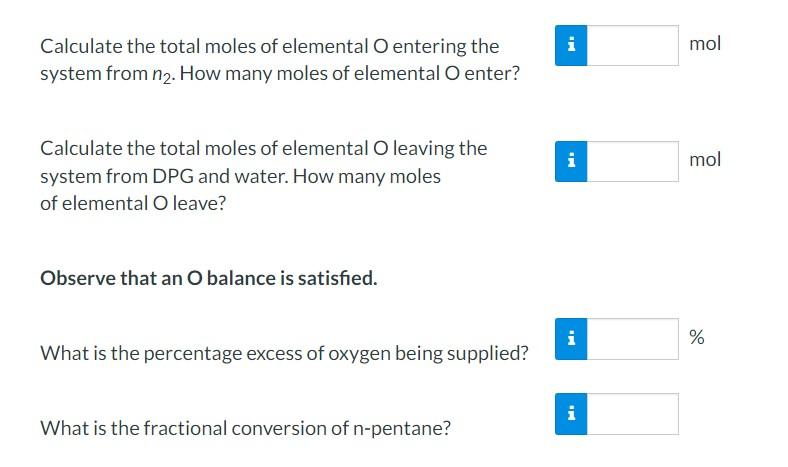
Calculate the total moles of elemental entering the system from . How many moles of elemental enter? Calculate the total moles of elemental leaving the system from DPG and water. How many moles of elemental O leave? Observe that an balance is satisfied. What is the percentage excess of oxygen being supplied? What is the fractional conversion of n-pentane?
Expert Answer
The balanced chemical equation for the combustion of n-Pentane with excess air is:C5H12+8O2?5CO2+6H2OFrom this equation,we can see that the stoichiome