Home /
Expert Answers /
Chemistry /
molarity-problems-calculate-the-molarity-of-the-following-solutions-abd-complete-the-soluble-abd-ins-pa447
(Solved): Molarity Problems Calculate the molarity of the following solutions abd complete the soluble abd ins ...
Molarity Problems Calculate the molarity of the following solutions abd complete the soluble abd insoluble salts lab. thank you


Molarity Problems Calculate the molarity of the following solutions: 1. 2.5 liters which contain 3.82 moles of sodium hydroxide. 2. 2.00 liters which contain 5.32 moles of bydrogen chloride. 3. 150 liter which contain 0.35 mole of potassium hydroxide. How many moles of are contained in: 4. 0.250 liter of a 2.0 molar solution. 5. of a 0.50 molar solution. 6. of a 150 molar 5 lution. How many liters of solution can be made from each of the following? 7. A solution of using 2.56 moles 8. A solution of s using 0.358 moles 9. A solution using 0.08 moles How many liters of solution can be made from each of the following? 10. A solution using of sodium hydroxide,
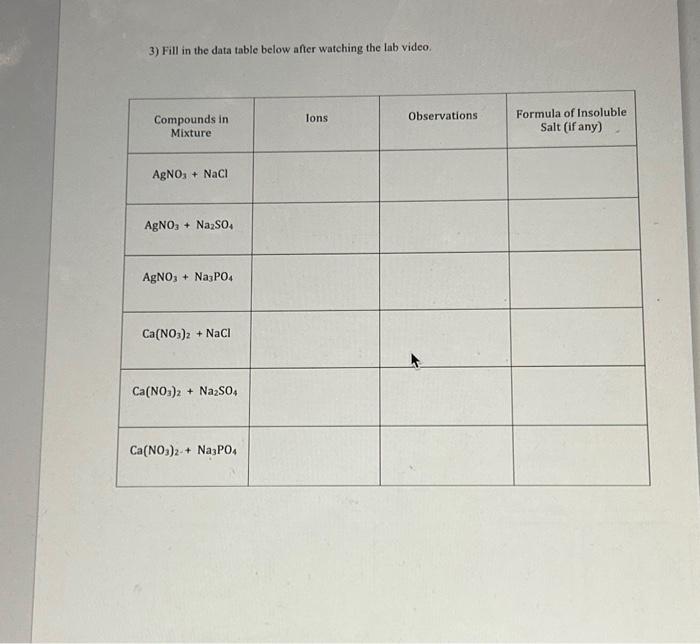
Soluble and Insoluble Salts Lab 1) Predict the products for the following reactions and balance each reaction. Include the states of matter for each product. Use this information along with the lab video to fill in the data table below. 1. 2. 3. 4. 5. 6. 2) What is an insoluble salt?
3) Fill in the data table below after watching the lab video.