Home /
Expert Answers /
Chemistry /
molar-ratios-with-unbalanced-equation-nbsp-molar-ratios-the-34-alum-34-used-in-cooking-is-potassium--pa597
(Solved): molar ratios with unbalanced equation Molar Ratios: The "alum" used in cooking is potassium al ...
molar ratios with unbalanced equation
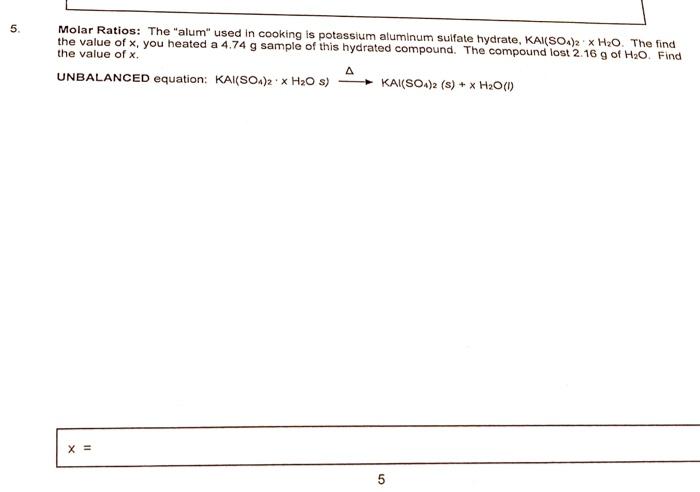

Molar Ratios: The "alum" used in cooking is potassium aluminum sulfate hydrate, \( \mathrm{KAl}\left(\mathrm{SO}_{4}\right)_{2} \cdot \times \mathrm{H}_{2} \mathrm{O} \). The find the value of \( x_{1} \) you heated a \( 4.74 \mathrm{~g} \) sample of this hydrated compound. The compound lost \( 2.16 \mathrm{~g} \) of \( \mathrm{H}_{2} \mathrm{O} \). Find the value of \( x \). UNBALANCED equation: \( \left.\mathrm{KAl}\left(\mathrm{SO}_{4}\right)_{2} \cdot \times \mathrm{H}_{2} \mathrm{O} \mathrm{s}\right) \stackrel{\Delta}{\longrightarrow} \mathrm{KAl}\left(\mathrm{SO}_{4}\right)_{2}(\mathrm{~s})+\times \mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \)